Some mammalian tissues regenerate efficiently after injury. This is the case of, for example, the skin or intestine, where the activation of tissue-resident stem cells leads to the replacement of cells lost to injury. The spinal cord is not one of them, and spinal cord injuries often lead to permanent functional impairment, as many cells lost to injury are never replaced.
Research over recent decades has demonstrated the existence of neural stem cells in the adult spinal cord. These cells are activated by injury, but produce, almost exclusively, scar-forming astrocytes, and the contribution of neural stem cells to the replacement of other cell types, such as neurons or oligodendrocytes, is markedly insufficient. Within our HFSP project, we set out to understand whether greater regenerative potential exists within resident spinal cord cells and whether we could recruit such potential.
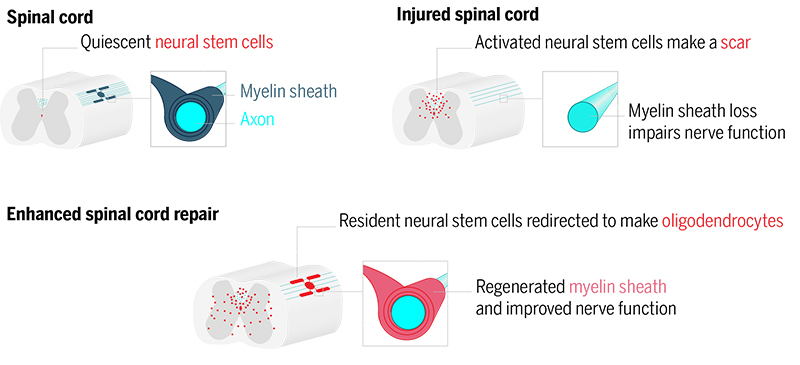
Figure: Recruiting resident neural stem cells for myelin repair. After spinal cord injury, myelin is lost near the injury site, which impairs axon function. In parallel, neural stem cells (ependymal cells, red) are activated but form a glial scar. Given the poised chromatin state in ependymal cells, overexpression of OLIG2 in ependymal cells in mice boosts oligodendrocyte production, which form new myelin and improve axon conduction.
The neural stem cell potential of the spinal cord resides in a well-characterized population of ependymal cells. We integrated single-cell RNA sequencing (scRNA-seq) and single-cell assay for transposase-accessible chromatin using sequencing (scATAC-seq) to study lineage potential in adult ependymal cells. We found that the genetic program for oligodendrocyte generation is accessible in ependymal cells. However, this program is latent, as oligodendrocyte genes are not expressed. In particular, we found that a large fraction of the genome where the transcription factor OLIG2 binds, had basal accessibility. This was intriguing because OLIG2 is the factor that initiates the production of oligodendrocytes during development, but OLIG2 expression is lost in adult ependymal cells. To study whether this latent accessibility could be harnessed to increase oligodendrocyte production from ependymal cells, we genetically engineered a mouse model to express OLIG2 in these cells. We found that OLIG2 expression was compatible with ependymal identity in the absence of injury. However, after a spinal cord injury, OLIG2 expression led to the increased accessibility of the latent oligodendrocyte program and subsequent expression of genes specifying oligodendrocyte identity. Activation of the latent program was followed by a boost in oligodendrocyte production.
Interestingly, the same manipulation failed to elicit oligodendrocyte production from astrocytes, suggesting a unique potential in the ependymal cell population. Using scRNA-seq of ependymal-derived cells, we found that new oligodendrocytes followed the developmental program of oligodendrocyte maturation, including an oligodendrocyte progenitor cell–like state that allowed the self-amplification of this newly recruited cell population. These cells later matured and became virtually indistinguishable from resident mature myelinating oligodendrocytes. Importantly, ependymal oligodendrocyte generation occurred in parallel and not at the expense of astrocyte scarring, which is itself necessary for proper wound closure. Newly recruited ependymal-derived oligodendrocytes migrated to sites of demyelination, where they remyelinated axons over the long term. Finally, we used optogenetics to measure axon conduction and found that ependymal-derived oligodendrocytes contributed to normalizing axon conduction after injury.
In this study, we have uncovered a hidden potential in adult neural stem cells. This lineage potential is normally not manifested, but targeted activation of such potential leads to the recruitment of neural stem cells for the generation of remyelinating oligodendrocytes in numbers comparable to those obtained via cell transplantation. We do not yet know whether these cells exist in sufficient numbers in adult humans or whether they will also be permissive to oligodendrocyte generation. However, our study indicates that resident cells may serve as a reservoir for cellular replacement and may offer an alternative to cell transplantation after CNS injury.


































