Most structural techniques rely on purification of membrane proteins using detergents, followed, in some cases, by reconstitution into synthetic lipid bilayers. Neither system can fully mimic the complex nature of these proteins’ natural environment, and the choice of mimetic can have significant impact on both structure and function. Therefore, it is desirable to study membrane proteins in their native membranes. Two methods which allow for structural investigations in native membranes are solid-state nuclear magnetic resonance spectroscopy (ssNMR) and electron cryotomography (cryoET).
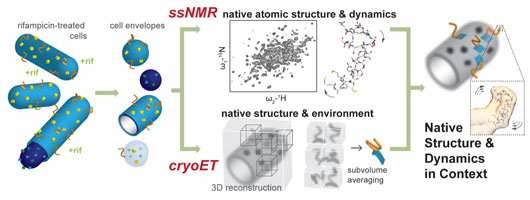
Figure: Our experimental approach uses isotopically labeled, rifampicin-treated bacteria that are gently disrupted to produce cell envelope particles. These cell envelope particles are subjected to 1H-detected magic angle spinning (MAS) solid-state NMR spectroscopy (ssNMR), which provides atomic structural and dynamical information, and electron cryotomography (cryoET), which provides environmental context and larger structural information through subvolume averaging. Combining information from these two techniques allows a structural and functional model to be built in native membranes.
CryoET and ssNMR provide highly complementary information. CryoET images individual events while ssNMR records bulk measurements. Motion and dynamics are implicitly included in ssNMR experiments, while cryoET provides snapshots on the seconds – hours timescale. Nanometer-scale spatial information is intrinsic to cryoET, while ssNMR is much more sensitive to chemical information on the Ångstrom scale. Further, ssNMR exploits isotope labeling to exclude background signals, whereas cryoET records the full environment. To take full advantage of this complementarity, as well as recent technological improvements in both methods, we designed an experimental system with a single sample, suitable for both techniques, that maintains the native membrane environment by avoiding the use of detergents or other extraction strategies altogether.
Our method allows the structure and function of bacterial membrane proteins to be studied in their native environments, across different spatial and temporal resolutions, and the combination is more powerful than each technique individually. We have used the method to study YidC, an inner membrane protein from Escherichia coli that helps fold and insert other inner membrane proteins, demonstrating that YidC adopts a different conformation in native membranes and that substrate binding to YidC in these native membranes differs from purified and reconstituted systems. These results emphasize the importance of maintaining the native environment of biological molecules when studying their structure and function.
HFSP funding was instrumental to this research, as it allowed us to explore methods other funding agencies might consider too risky, and helped combine highly specialised expertise from laboratories in different fields and different countries.
Reference
Combined 1H-detected solid-state NMR spectroscopy and electron cryotomography to study membrane proteins across resolutions in native environments. Baker, L.A., Sinnige, T., Schellenberger, P., de Keyzer, J., Siebert, C.A., Driessen, A.J.M., Baldus, M., and Grünewald, K. Structure. 2018; 26: 161–170


































