Evolution has perfected many mechanisms for fitness and survival, but perhaps the most visually impressive phenomenon is bioluminescence, the generation of “cold light”—illumination by living organisms without energy dissipation as heat. Thousands of both marine and terrestrial organisms including bacteria, ostracods, fireflies, dinoflagellates, cnidarians, worms, shrimps, snails, sharks and octopi, among others, use light to communicate warning signals, lure prey or attract partners for mating. Within the core of the bioluminescence phenomenon lies fascinating photochemistry that converts the energy stored within the chemical bonds into bright light, similar to the reactions between two solutions used in glow-sticks. In bioluminescence, through an otherwise electronically forbidden process, a chemical reaction within an enzyme (luciferase) converts a ground-state molecule carrying the mystic name luciferin (derived from “light bearer”) to the excited state of the respective oxidized product, called oxyluciferin. Depending on its chemical state and interactions with the environment, the excited state of oxyluciferins can emit a myriad of colors ranging from bright blue, which is observed mostly with marine organisms, to yellow-green and glowing red with many of the terrestrial bioluminescent organisms.
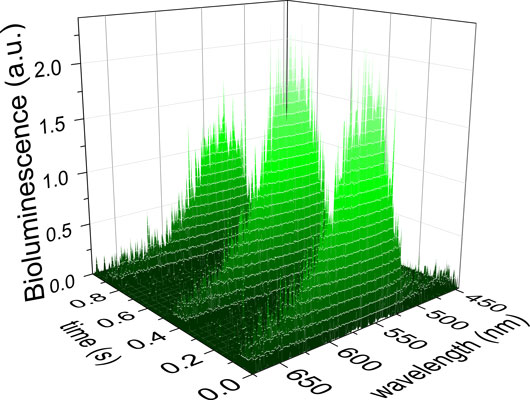
Figure: Broadband bioluminescence of an agitated firefly recorded in vivo.
From the natural bioluminescence reactions with an exogenous (external) substrate, the firefly bioluminescence tops the list of accessible fluorescence yields. Yet, there is no consensus yet as to the exact chemical structure of the excited oxyluciferin, which fireflies utilize to emit light. Early studies have suggested an enolate-ion as the reactive species, although the generally accepted mechanism for the bioluminescence reaction sequence implies a keto-ion. Moreover, by deliberate point mutations, the emission of the enzyme that catalyzes the bioluminescence reaction can be artificially tuned between green and red, and the reasons by which the luciferases can shift the light across this bioluminescent rainbow remain elusive. A dozen or so mechanisms have been advanced to explain the molecular origin of this phenomenon and many of them were later refuted based on theoretical or experimental grounds.
The firefly oxyluciferin is a structurally simple, yet chemically intricate molecule. It can exist as a mixture of up to six acid-base forms, and some of them are capable of photoinduced proton transfer reactions. Our early efforts succeeded in establishing the crystal structure of the oxyluciferin,1 and provided the preliminary information on its complex spectrochemistry,2 but the research was discontinued due to a lack of funding. The collaborative HFSP-sponsored project between New York University, Technical University of Munich, University of Lille 1 and University of Strasbourg, together with collaborators from other institutions, has helped continue this research, and in turn it has now tremendously advanced the burgeoning research on firefly bioluminescence. By using mathematical modelling (MCR-ALS), the lifetimes and the absorption and emission spectra of the chemical forms of the emitter were disentangled.3,4 Additionally, the solid-state spectral signature of its chemical and isotopic variants were investigated.5 Action spectroscopy of the lumophore in vacuo revealed that its electronic states were altered by microenvironmental perturbations.6 The results also provided evidence of the excited-state tautomerization, which is the key to resolving the enol−keto conundrum related to the color-tuning mechanism.7 The reasons for the apparent instability of oxyluciferin were also explained.8
The main obstacle to fully understanding the chemical form of oxyluciferin has been the lack of knowledge on its excited-state dynamics in the active site of the luciferase. The complex mixtures of up to six interconvertible chemical forms, the dependence of the chemical equilibria on the solvent composition and pH, and the proclivity of the emitter for dimerization in basic conditions pose major hurdles for modeling the spectrochemistry of oxyluciferin. In the most recent contribution,9 which is a collaborative project between New York University Abu Dhabi (P. Naumov), Georgia Institute of Technology (K. M. Solntsev), University of East Anglia (S. Laptenok), VU University Amsterdam (J. J. Snellenburg) and Olis Inc. (R. J. DeSa, K. M. Solntsev), we mimicked the naturally occurring firefly bioluminescence by studying the oxyluciferin as a complex with the luciferase from the Japanese firefly (Luciola cruciata). Because of the extremely complicated nature of the system, it was necessary to do a simultaneous global and target analysis of multiple experiments at various pH for two excitation wavelengths. Assuming that the spectral and kinetic properties of the different chemical forms of the chromophore were independent of the pH, a single unifying model was developed. The results of the modelling revealed a much more detailed and accurate picture of this multichromophoric system under conditions that are directly applicable to the real system. These results also demonstrated the relevance of the photoinduced proton transfer dynamics for the color tuning of firefly bioluminescence. Finally, the results of the analysis were compared to the time-resolved bioluminescence recorded for the first time in vivo from a North-American firefly. These results provide direct insight into the photochemistry behind the mysterious flashes of fireflies, and set the stage towards a better understanding of color modulation by other bioluminescent organisms.
References
[1] Structure and Spectroscopy of Oxyluciferin, the Light Emitter of the Firefly Bioluminescence, Naumov, Panče; Ozawa, Yutaka; Ohkubo, Kei; Fukuzumi, Shunichi, Journal of the American Chemical Society (2009) 131(32), 11590—11605.
[2] Spectra-Structural Effects of the Keto-Enol-Enolate and Phenol-Phenolate Equilibria of Oxyluciferin. Naumov, Panče; Kochunnoonny, Manoj, Journal of the American Chemical Society (2010) 132, 11566–11579.
[3] Deciphering the Protonation and Tautomeric Equilibria of Firefly Oxyluciferin by Molecular Engineering and Multivariate Curve Resolution. Rebarz, Mateusz; Kukovec, Boris-Marko; Maltsev, Oleg V.; Ruckebusch, Cyril, Hintermann, Lukas, Naumov, Pance; Sliwa, Michel, Chemical Science (2013) 4, 3803—3809.
[4] Emission Properties of Oxyluciferin and its Derivatives in Water: Revealing the Nature of the Emissive Species in Firefly Bioluminescence. Ghose, Avisek; Rebarz, Mateusz; Maltsev, Oleg V.; Hintermann, Lukas; Ruckebusch, Cyril; Fron, Eduard; Hofkens, Johan; Mély, Yves; Naumov, Panče; Sliwa, Michel; Didier, Pascal, Journal of Physical Chemistry B (2015) 119, 2638—2649.
[5] Vibrational Spectra of Chemical and Isotopic Variants of Oxyluciferin, the Light Emitter in the Firefly Bioluminescence. Maltsev, Oleg V.; Yue, Ling; Rebarz, Mateusz; Hintermann, Lukas; Sliwa, Michel; Ruckebusch, Cyril; Pejov, Ljupčo;* Liu, Ya-Jun; Naumov, Panče, Chemistry – A European Journal (2014) 20, 10529—10837.
[6] On the Influence of Water on the Electronic Structure of Firefly Oxyluciferin Anions from Absorption Spectroscopy of Bare and Monohydrated Ions in Vacuo. Støchkel, Kristian; Nygaard Hansen, Christian; Houmøller, Jørgen; Munksgaard Nielsen, Lisbeth; Anggara, Kelvin; Linares, Mathieu; Norman, Patrick; Nogueira, Fernando; Maltsev, Oleg V.; Hintermann, Lukas; Brøndsted Nielsen, Steen; Naumov, Panče; Milne, Bruce F., Journal of the American Chemical Society (2013) 135, 6485—6493.
[7] Photoinduced Dynamics of Oxyluciferin Analogues: Unusual Enol “Super”photoacidity and Evidence for Keto−Enol Isomerization. Solntsev, Kyril M.; Laptenok, Sergey P.; Naumov, Panče, Journal of the American Chemical Society (2012) 134, 16452—16455.
[8] Why is Firefly Oxyluciferin a Notoriously Labile Substance? Maltsev, Oleg V.; Nath, Naba K.; Naumov, Panče; Hintermann, Lukas, Angewandte Chemie, International Edition (2014) 53, 847—850.
[9] Excited-State Dynamics of Oxyluciferin in Firefly Luciferase, Snellenburg, Joris J.; Laptenok, Sergey P.; DeSa, Richard J.; Naumov, Panče; Solntsev, Kyril M. Journal of the American Chemical Society (2016) 138, 16252—16258.
Guiding light: the mysteries of firefly bioluminescence unfold by Pance Naumov


































