To carry out cellular-level circuit analysis in intact animals, single-neuron monitoring and manipulation are needed. Intra-cellular recordings and current injection can achieve this, but that approach is not scalable, especially in freely-moving animals. Optical methods can combine single-cell resolution with single-cell targeting, but they are limited to a few hundred microns from the brain surface. Individual neurons can be isolated using extracellular recording, and optogenetic manipulations can achieve high temporal resolution. However, methods that combine extracellular recordings with optical stimulation typically use light guides that require tethering of the animal with glass fibers, limiting the freedom of movement, and emit much more light than is required to activate a single neuron.
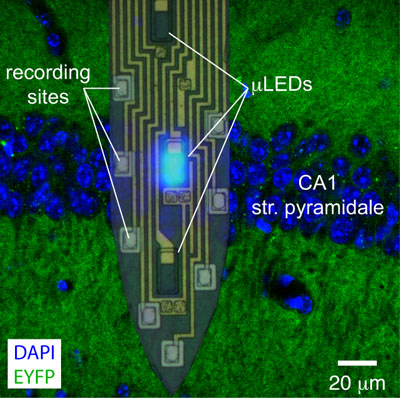
Figure: A single shank of a four shank (32-site/12-LED) micro-LED probe is shown overlaid on a confocal image of the mouse CA1 pyramidal cell layer (EYFP expressed under the CaMKII promoter). With proper localization, distinct micro-LEDs illuminate tissue below, in, or above the layer, while spiking of more than a dozen neurons is monitored.
To solve these issues, we decided to take two conceptual steps. First, instead of using light sources on the bench, we designed devices in which light sources are implanted in the brain: neuron-sized micro-light-emitting-diodes (micro-LEDs). Second, instead of having the light sources and the electrodes manufactured separately and then possibly coupled, we manufactured them on the same monolithic device. These two innovations yielded devices (micro-LED probes) that have neuron-sized light sources interspersed between neuron-sized electrodes. The only connection to the probes is electrical, and thus free animal movement is permitted. The active part of the probe spans less than 200 micro-meters, and the recording shanks span 5 mm, and thus these probes can control neuronal activity deep in the rodent brain.
We implanted micro-LED probes in the hippocampus CA1 layer of mice that expressed ChR2, a blue-light sensitive opsin, specifically in pyramidal cells (see Figure). Using less than 100 nano-Watt of blue light (460 nm), we were able to drive the spiking of individual neurons, and to independently control spiking of cells about 50 micro-meters apart. Higher light intensities used at the bottom or the top of the layer induced distinct high-frequency (~120 Hz) generators to oscillate. The scalability and spatiotemporal resolution of this tool provides versatility and precision for cellular-level circuit analysis in deep structures of intact, freely moving animals.
Reference
Monolithically integrated mLEDs on silicon neural probes for high-resolution optogenetic studies in behaving animals. Wu F, Stark E, Ku P, Wise K, Buzsáki G, Yoon E (2015) Neuron, 88:1136-1148.


































