The maintenance of spatial information contained within the architecture of the microtubule network is therefore intrinsically limited by the dynamics of microtubule regeneration. However, the most recent studies by the CytoMorpho laboratory published in the journal Nature Cell Biology (Aumeier et al, 2016), have shown that when microtubule structure is disrupted as a result of mechanical constraints or faulty assembly, the microtubules in those areas of the cell are protected from disassembly, and their half-lives extended. This protective process upsets the random dynamics of the microtubule network, and tends to lead to a more extensive network in those areas of the cell put under severe strain.
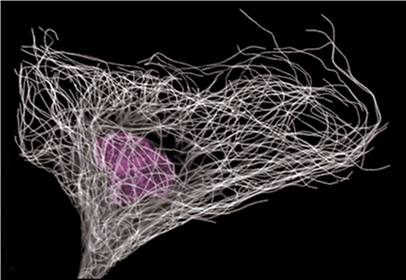
Figure 1: Immuno-labelling of microtubules (white) and nucleus (violet)
The process of constant regeneration enables the microtubule network to change its architecture and accompany the morphological changes of cells. This process of dynamic adaptation remains poorly understood. Tubulin is the building block, and its polymerisation leads to the assembly of the microtubule. The use of fluorescent tubulin allows the visualisation of the dynamics of microtubule assembly and disassembly in living cells. With this approach, microtubules can be shown to grow in a regular manner and that their growth can be randomly interrupted leading to rapid disassembly and disappearance. It is not uncommon to see the disassembly process suddenly interrupted. This interruption of microtubule disassembly, termed rescue event, enables the microtubule to resume growth and not disappear. Although the processes regulating microtubule assembly and disassembly have been described, very little is known about the rescue events. In fact, when tubulin is purified and used to re-enact the dynamics of assembly in vitro under simple biological conditions, assembly and disassembly occur normally but rescue events do not and the microtubules undergo complete depolymerisation. Surprisingly, CytoMorpho scientists discovered that by damaging the microtubule structure using laser beams, they were able to induce microtubule rescue in vitro.
Microtubule dynamics can be shown using kymographs, constructed by superimposing images of a given microtubule over time (Figure 2).

Figure 2: Kymographs
The kymograph images in Figure 3 show microtubules in vitro without treatment (coloured blue and on the left), and microtubules which have been targeted by laser beams (coloured green and on the right). The darkened areas running vertically down the kymograph images correspond to the laser impacts. The laser impacts act as protection against depolymerisation. By allowing assembly to resume, the rescue events increase the length and half-life of the microtubules. Charlotte Aumeier observed the same effect in living cells: the network of microtubules becomes more stable and extends into the areas where microtubule structure was damaged by laser impact. To paraphrase Nietzsche, what does not kill the microtubules makes them stronger.

Figure 3: Kymographs showing microtubules without treatment (blue) or following laser treatment (green)
How do these impacts which damage microtubule structure lead to resistance to disassembly?
It seems that the answer to this question lies in the capacity of microtubules to auto-repair. This extraordinary property, recently revealed by CytoMorpho scientists in Nature Materials (Schaedel et al, 2015), allows microtubules to react against mechanical constraints. It seems this property also increases the microtubule half-lives. By using two colours to visualise and differentiate molecules of tubulin incorporated into the microtubules from those free in solution, CytoMorpho scientists discovered that each laser impact on a microtubule was immediately repaired by molecules of free tubulin and that the areas with new tubulin molecules act as protective shields preventing depolymerisation and inducing rescue events.
Microtubule injuries other than laser impacts may lead to repair and rescue events. They occur naturally throughout the cell, in particular in areas where microtubules are deformed, such as where they intersect or form bundles (Figure 4). Due to the capacity to auto-repair using new components, the injuries constitute a fountain of youth for microtubules.
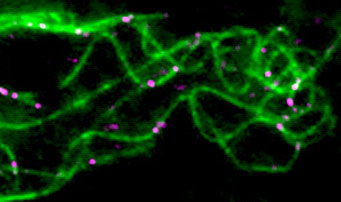
Figure 4: Microtubule repair by free tubulin (violet)
Furthermore, these processes of mechanical strengthening and increased stability confer the microtubule network with previously unknown properties of adaptation to mechanical constraints. In fact, a series of laser impacts targeting a specific area of the cell are sufficient to locally stabilise and make the network of microtubules more extensive in this area. The microtubule network interacts with all structures regulating cell shape, and the local elaboration of microtubules in response to damage thus affects cell shape. Remarkably, CytoMorpho scientists have been able to guide the movement of the cell by manipulating local networks of microtubules through repeated laser beam impacts.
By building on these observations, CytoMorpho scientists are keen to study in detail, the molecular mechanisms of microtubule auto-repair and how microtubules stabilise their growth. Certain cell types may depend to greater or lesser degrees on these mechanisms. For example, for neuronal microtubules, which have particularly long half-lives and play a major part in the transport of information, the question arises as to whether the mechano-sensation and adaptation of microtubule-network architecture are implicated in the regulation of neuronal functions.
Finally, as the components of the majority of biological structures are constantly being turned over, it is tempting to present the hypothesis that the mechanisms of adaptation and repair-reinforcement, highlighted by these recent studies on microtubules, are a general feature of biological structures in living organisms. In other words, damaging an inanimate object weakens it but damaging a biological structure can, paradoxically, strengthen it.
References
[1] Self-repair promotes microtubule rescue. Charlotte Aumeier, Laura Schaedel, Jérémie Gaillard, Karin John, Laurent Blanchoin and Manuel Théry. Nature Cell Biology, 18:1054–1164, 2016.
[2] Microtubules self-repair in response to mechanical stress. Laura Schaedel, Karin John, Jérémie Gaillard, Maxence Nachury, Laurent Blanchoin and Manuel Théry, Nature Materials, 14:1156–1163, 2015.


































