Every living being experiences the daily rotation of earth around its axes, which generates the alternation of day and night. Organisms adapt to this 24 hours cycle thanks to an extremely specialized circadian (from Latin: circa diem, around the day) clock present in their body and able to control vital processes. Indeed, many biochemical, behavioural and physiological processes, such as sleep-wake cycle, blood pressure, hormonal levels etc., are under circadian rhythmicity. It is clear that circadian clocks are present in every tissue and contribute to maintaining the physiology of that tissue. For instance, the liver clock system plays a crucial role in controlling our metabolism and energy homeostasis. Moreover, food and feeding behaviour are the most important signals responsible for the synchronization of the liver clock. Thus, changes in dietary habits, such as the consumption of food rich in fat, can perturb the functions of the liver clock and in consequence of that hepatic physiology and whole body metabolic homeostasis.
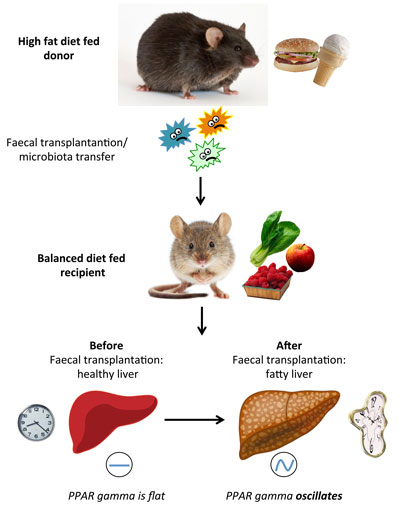
Figure: Schematic representation of the faecal transplantation experiment. The intestinal microbes from high fat diet-fed donor mice were transplanted into balanced diet-fed recipients through oral gavage. Two weeks after the faecal transplantation the liver of recipient mice became fatty and the transcription factor PPAR-gamma and its target genes started to oscillate in a circadian manner.
Our body has an intricate mutualistic relationship with a myriad of microorganisms populating all its surfaces in contact with the external environment. These microbes are particularly abundant in the intestine in which they constitute the so-called “gut microbiota” or “gut flora”. The gut microbiota plays a key role in the development of the host immune system, in preserving correct metabolic functions and it can even influence the behaviour of the host. Notably, diet is one of the major determinants of the gut flora composition and food rich in fat can alter the ecosystem causing a microbial imbalance (i.e. dysbiosis).
Paola Tognini and colleagues in Sassone-Corsi’s lab at the University of California, Irvine tried to understand how diet-induced alterations in the gut microbiota composition could influence the circadian clock in a distant tissue: the liver. Donor mice were fed a high fat diet or a balanced diet (control chow) and their fresh faeces were collected. The recipient mice were all fed a control chow and were subjected to faecal transplantation through oral gavage. The recipient mice receiving faeces from high-fat fed donors experienced an increase in body fat mass and fatty liver, even though they were eating a healthy chow. Moreover, the liver gene expression along the day showed dramatic differences with respect to the control recipient. In particular, a specific group of genes involved in lipid metabolism that has generally a constant expression at different times of the day, started to oscillate in a circadian manner in the liver of high fat recipient animals. Remarkably, these genes were targets of a specific transcription factor implicated in fatty acid storage and glucose metabolism: the nuclear receptor PPAR-gamma, whose nuclear accumulation became oscillatory as well. Thus, the transfer of the gut flora of high fat fed mice perfectly mimicked the liver circadian clock changes driven by high fat feeding. The pharmacological inhibition of PPAR-gamma completely blocked the high fat faeces transplantation effect both on fat mass increase and gene expression induction, suggesting that PPAR-gamma is the major player translating the signals from the intestinal flora to the liver clock.
Finally, to demonstrate that the gut microbiota dysbiosis was necessary for the effect of high fat diet on the liver clock, mice were fed a high fat diet and simultaneously treated with an antibiotic cocktail to deplete the intestinal bacteria. Strikingly, the antibiotic treated mice were not gaining as much weight as the control mice and in the liver the PPAR-gamma target genes circadian oscillation was totally prevented, indicating the importance of the gut microbes’ involvement in hepatic physiological and molecular changes in response to a nutritional stress.
In summary, our study demonstrates that the rewiring of the liver circadian transcriptional landscape induced by high fat feeding is mediated by signals coming from the intestinal microbiota that participate to the activation of specific molecular cascades. Elucidating these pathways could contribute to the development of a new pharmacological strategy to treat metabolic syndrome and obesity.
Reference
“Gut microbiota directs PPARγ-driven reprogramming of the liver circadian clock by nutritional challenge”. Murakami M.*, Tognini P.*, Liu Y., Eckel-Mahan K.L., Baldi P., Sassone-Corsi P. EMBO reports, 17 (2016) 1292-1303.


































