When C. elegans measures a very large or a very fast change in temperature, the worm will perform a stereotyped escape response. It will quickly move backwards for many body lengths and then make a deep body bend to reorient itself away from potential harm. While some of the genes and neurons involved in the thermal noxious response have been identified, there are many gaps that need to be filled in before we have a complete behavioral microcircuit.
Finding new components or mechanisms of behavioral circuits is time-consuming due to the low efficiency of currently available functional screening techniques. To overcome such obstacles, we utilized a pan-neuronal approach to broadly screen the activity of the C. elegans nervous system in response to thermal stimuli. We did this by building strains which genetically express calcium indicators localized to all their neuronal nuclei. The nuclear localization helped us with the computationally difficult step of neuronal segmentation and identification.
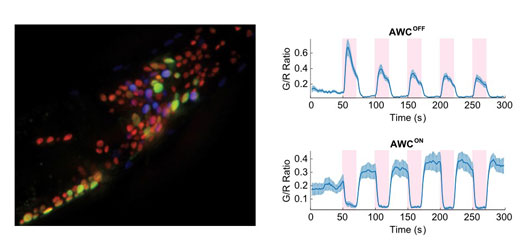
Figure: Whole “brain” recording of C. elegans and activity measurements of AWC in response to temperature increase (pink boxes).
Using pan-neuronal screening, we discovered that the calcium flux in a pair of AWC neurons respond in opposite directions in reaction to noxious heat stimulation. AWCs are sensory neurons located in the head region of C. elegans, and are crucial for sensing certain volatile chemicals. Unlike most other pair-wise neurons, AWC neurons are functionally asymmetric: the AWCON neuron senses butanone odor, and AWCOFF senses 2,3-pentanedione, while both neurons symmetrically sense benzaldehyde and isoamyl alcohol which are essential for proper chemotaxis.
When we applied either a very fast temperature rise (a few degrees in less than 50 ms) or high absolute temperature (33°C), we detected very deterministic AWC signals that are asymmetric in AWCON and AWCOFF: the calcium transients in AWCOFF neurons are always positively correlated to the nociceptive thermal stimuli whereas the transients in AWCON neurons are negatively correlated. This novel activity was then tested in freely moving animals in which the nociceptive stimulation was applied to further investigate their role in physiological behaviors utilizing asymmetry mutants and laser ablation of AWCs. Indeed, the thermal avoidance behavior, which has been linked to the activities in the AFD, FLP, PVD, and PHC neurons is also strongly coupled to the AWCOFF neurons but not AWCON. By combining pan-neuronal calcium imaging with our novel behavioral analyses, we found that the AWCOFF neuron is essential and sufficient for noxious heat sensation and the subsequent avoidance behavior.
This work is a natural continuation of two previously HFSP funded collaborations. The first was to understand how worms perform searches in the absence of thermal signals (1) and the second was to model the perceived levels of thermal stimuli from the worm’s body language in response to thermal “pain” (2).
Reference
Pan-neuronal screening in Caenorhabditis elegans reveals asymmetric dynamics of AWC neurons is critical for thermal avoidance behavior. Kotera I, Tran NA, Fu D, Kim JH, Byrne Rodgers J, Ryu WS. Elife. 2016 Nov 16;5. pii: e19021. Doi: 10.7554/eLife.19021. PubMed PMID: 27849153; PubMed Central PMCID: PMC5142811.
Other references
1. Foraging success under uncertainty: search tradeoffs and optimal space use. Bartumeus F, Campos D, Ryu WS, Lloret-Cabot R, Méndez V, Catalan J. Ecol Lett. 2016 Nov;19(11):1299-1313. doi: 10.1111/ele.12660. PubMed PMID: 27634051.
2. Stereotypical Escape Behavior in Caenorhabditis elegans Allows Quantification of Effective Heat Stimulus Level. Leung K, Mohammadi A, Ryu WS, Nemenman I. PLoS Comput Biol. 2016 Dec 27;12(12):e1005262. Doi: 10.1371/journal.pcbi.1005262. PubMed PMID: 28027302; PubMed Central PMCID: PMC5189946.


































