Work over the past several decades has demonstrated that cortical assemblies, groups of neurons in the cerebral cortex which tend to coactivate, form fundamental functional units necessary for the performance of cognitive tasks such as learning. However, the more general question of their role in consciousness is unclear. In this study, Richard Boyce, HFSP Long-term Fellowship Awardee 2018, developed an approach combining imaging of local cortical neural activity with large scale EEG recordings in naturally sleeping mice, revealing valuable insight into the neural correlates of consciousness.
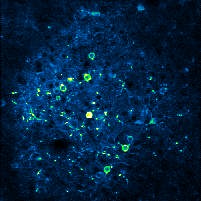
The experimental approach employed by the researchers was initially centered around the use of 2-photon calcium imaging for observing local neural activity (microcircuit level) from the sensorimotor cortex in mice that had been trained to sleep naturally under a microscope. The first step involved isolating recordings of cortical activity during different natural vigilance states (active wakefulness, quiet wakefulness, non-REM sleep, and REM sleep) to allow for analysis of cortical neural dynamics during different behavioral states. Although cortical assemblies were found to be larger during periods of active wake, there were no differences in the characteristics of cortical assembly activity during periods of quiet wakefulness, non-REM sleep, or REM sleep, despite the latter two vigilance states being associated with significantly reduced levels of consciousness. While this initial result could be seen to be inconsistent with the idea that cortical assemblies play a fundamental role in consciousness, prior studies using comparatively coarse measures of local neural activity (e.g., local EEG) suggested that medically induced loss of consciousness (anesthesia induction) was often associated with relatively intact local patterns of brain activity, although the coordination of this activity between distant brain regions was disturbed. The researchers therefore next hypothesized that there might be a difference in the relationship between local and global brain activity patterns as a function of brain state which could provide an explanation for their seemingly paradoxical initial results. To evaluate this possibility, the team incorporated multiple EEG electrodes spread all over the cortex - which allows for the assessment of global brain dynamics using an established approach called ‘EEG microstate analysis’ - into the 2-photon calcium imaging experimental setup. Consistent with their revised hypothesis, local cortical assembly activity was subsequently found to be coordinated with global brain activity dynamics during wakefulness, but not sleep, when consciousness is relatively reduced.
These results provide valuable insight into the neural correlates of consciousness by suggesting that the coordination of local cortical activity with global brain dynamics may be a key mechanism for sustaining consciousness during waking states, perhaps by facilitating the temporary binding of cortical assembly activity across relatively distant brain regions. Boyce and colleagues are currently working to extend these results in humans via analysis of data obtained through collaboration. Considering that alteration of global brain dynamics is a hallmark of several prevalent conditions, including Schizophrenia, they are also looking to evaluate whether disturbed coordination of cortical assemblies with global brain activity patterns could be associated with pathological states. For the researchers, the long-term hope is that these experiments could lead to the identification of new therapeutic targets for currently uncurable neuropsychiatric disorders.


































