Our muscles power our daily movements. The force is produced by large macro-molecular assemblies called sarcomeres. Many of these 3 µm long sarcomeres are chained together into long periodic myofibrils that span across millimeter long muscle cells. A long-standing question in the muscle field is how the highly ordered sarcomeres arise from their initially unordered components during muscle development.
An interdisciplinary team of biologists from the Schnorrer group in Marseille has now teamed up with physicists based in the Friedrich group in Dresden to to sketch a mathematical model of how actin filaments, myosin filaments, and the cross-linking titin proteins may assemble into the first observable periodic sarcomeres in muscle cells. The modeling path was guided by the experimental observation that myosin and the large titin protein, which links myosin to proteins at the sarcomere border called the Z-disc, form a periodic pattern first. To the surprise of the HFSP Grant researchers, the actin filaments are sorted into their well-known periodic pattern only with some delay in the here investigated model muscle, the flight muscles of Drosophila melanogaster.
The HFSP Awardees developed a mathematical model in which sarcomere proteins bind and unbind to a disordered bundle of actin filaments. Positive feedback allows myosin to recruit more myosin, and a titin length component keeps the Z-disc at a distance from myosin. Such conditions can spontaneously self-assemble a periodic sarcomere pattern. Perturbing protein concentrations in the computer simulations allows to make predictions for future experiments.
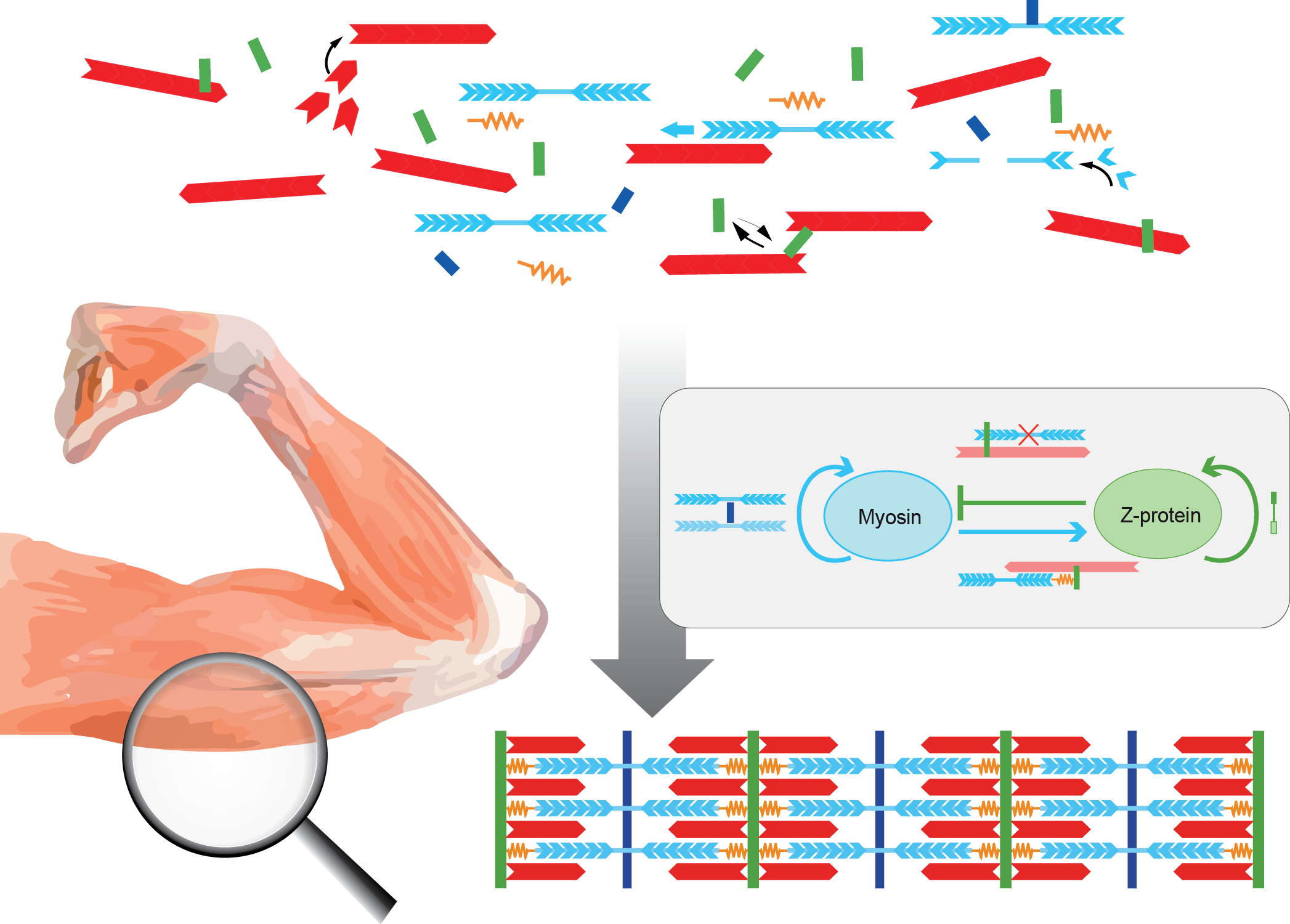
The Schnorrer group had shown earlier that in vivo sarcomeres can only assemble when they experience mechanical tension. Thus, the Friedrich group adapted their first model further by proposing that binding between proteins becomes stronger when they experience tension, forming so-called catch-bond crosslinkers. This tension is produced by the molecular motor myosin, a key component of the sarcomere. This mechanical feedback can further contribute to the formation of periodic patterns, as seen in muscles in vivo.
In conclusion, by combining experiment and theory, the groups provide new molecular insight into how regular sarcomeres assemble during muscle development. According to the researchers, the interdisciplinary and cross-country HFSP funding was pivotal to kick-start this collaboration between Marseille and Dresden: "It not only funded the experimental and theoretical lab work but, importantly, also allowed for regular lab visits, both online and in person, a key asset in particular during the pandemic time."


































