The plant cell, like any eukaryotic cell, is able to perceive environmental signals and transduce them to the nucleus, which in turn modify the cell response (1). Until now, the nuclear response was mostly analyzed from the biochemical and transcriptional points of view. But nuclei are also physical objects and their mechanics likely play an important role in signal transduction.
To modify the mechanical status of the nucleus, we used salt and sugar to mimic drought conditions, a physiological stress, in the model plant A. thaliana. Visualizing a marker of the nuclear envelope by live cell imaging, we found that the nucleus changes its shape in the root tip cells. Using micro-rheometry, this was correlated with increased nucleus stiffness and compactness under such hyperosmotic stress.
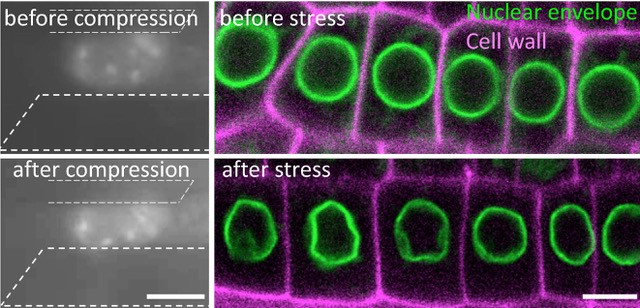
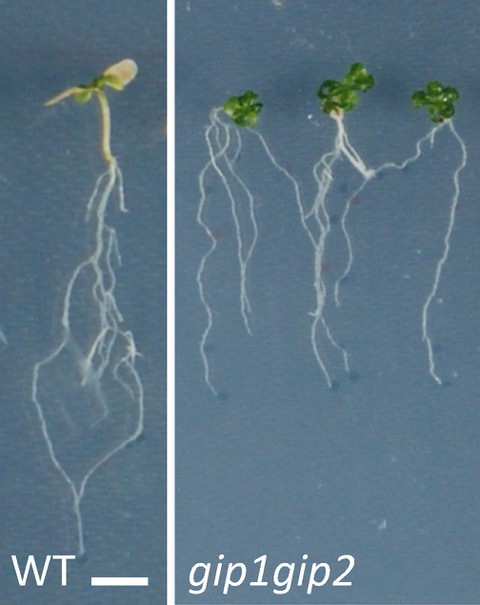
This response was associated with the induction of mechanosensitive genes that allow the plant to resist stress. All these responses being reversible, we show that the nucleus behaves like a mechanical rheostat in plants. The small multifunctional proteins GIPs/MZT1 (2), located in particular on both sides of the nuclear envelope, and interacting with the microtubule cytoskeleton in the cytoplasm (3) and with the centromeres in the nucleus (4), negatively regulates these responses. Mutants deficient in GIPs exhibit a constitutive response to hyperosmotic stress. In other words, in normal osmotic conditions, the nuclei of the mutant's cells are already deformed and prepared to resist stress. They give plants greater resistance to hyperosmotic stress, in particular by limiting the effects of senescence on their leaf system. These results open a new avenue of research on the role of the nuclear envelope in the perception of environmental and mechanical stresses, and allow a better understanding of how plants resist water stress.
The support of HFSP in this work has been instrumental to set the basis of nuclear mechanics investigation and to further explore the link between nuclear shape and chromatin remodeling through mechano-transduction at the single cell level.