Fibroblasts synthesize collagen and other extracellular matrix (ECM) molecules to promote wound healing. This program is hijacked in tumors, resulting in aberrant ECM accumulation that supports various aspects of tumor progression, including tumor growth, metastasis and therapy resistance.
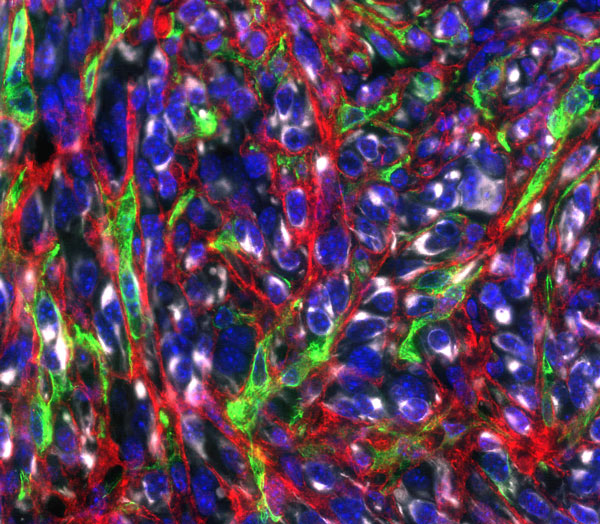
Figure: In pancreatic tumors, cancer cells (white) are embedded in a collagen matrix (white) produced by cancer-associated fibroblasts (green) that supports tumor growth. Nuclei are shown in blue.
Credit: Simon Schwörer.
In previous work (Schwörer et al, 2020), we identified core metabolic pathways that allow fibroblasts to generate the building blocks required to synthesize ECM. These building blocks (amino acids) are made from glucose and glutamine, the major nutrient sources for cells. Glucose and glutamine are oxidized in the TCA cycle, and this process allows fibroblasts to synthesize amino acids that are specifically enriched in the ECM, such as proline.
As tumors grow in size, they often outstrip their vascular supply. As a result, blood-derived nutrients including glucose and glutamine have been found to be limiting in tumors. Paradoxically, collagen and other ECM molecules are enriched in such tumors and continue to support their growth. To understand the mechanisms that allow cancer-associated fibroblasts (CAFs) to continue synthesizing ECM in tumors, we mimicked the nutrient environment of tumors in culture, and analyzed the activity of metabolic pathways that feed into the TCA cycle. This analysis identified the enzyme pyruvate carboxylase (PC) to be activated in CAFs under nutrient-poor conditions. PC was required for the synthesis of collagen and other ECM molecules in nutrient-poor, tumor-like conditions, but not in nutrient-rich conditions. When we impaired PC specifically in CAFs, pancreatic and breast tumors accumulated less collagen and tumors grew significantly slower.
We further found that the PC-catalyzed reaction in CAFs is not fueled by glucose-derived pyruvate. Instead, CAFs use pyruvate derived from the uptake of the metabolic waste product lactate, which can accumulate in tumors due to the metabolic activity of cancer cells and the impaired transport out of tumors due to vascular insufficiency. This helps explain how CAFs can maintain TCA cycle activity and the synthesis of building blocks for ECM production even when glucose and glutamine are limiting.
Taken together, our results demonstrate that cancer-associated fibroblasts are metabolically flexible, which allows them to sustain their tumor promoting activities even when their canonical nutrient sources are deprived. They also highlight a novel metabolic node that could be targeted to impair CAF-mediated extracellular matrix synthesis selectively in tumors.
|
HFSP award information Long-Term Fellowship (LT000854/2018-L): Deciphering metabolic rewiring of cysteine metabolism for redox control in cancer cells Fellow: Simon Schwörer |


































