But can we truly optimize a ~3.5 billion year old process? Several indications argue that yes, we can. First, modern agriculture has altered the conditions in which plants carry out photosynthesis (denser canopies, shorter growth cycles, etc.). Second, there has been continued selection pressure due to everchanging conditions (atmospheric CO2, temperature, rainfall). Further, there is ample support for the untapped potential of this approach, from numerous cases where replacement or modification of a single gene or pathway had profound effects on plant biomass yield and photosynthetic performance (see [1-7]).
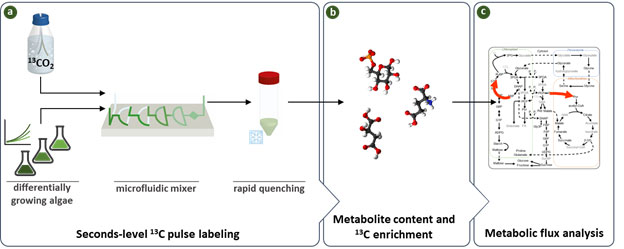
Figure: Schematic of the workflow employed in this study.
(a) An experimental setup to provide precise short pulses to algal cultures differing in their growth rate based on microfluidic mixer and instantaneous quenching.
(b) An analytical setup to measure levels and labeling kinetics of intermediates in the CBC and the early steps of the end-product synthesis pathways.
(c) Inspection of labeling temporal kinetics to identify differential flux patterns between algal species.
The theoretical limit of photosynthesis’ efficiency (defined as the fraction of light energy that is captured in conversion of CO2 and water to glucose) is widely accepted as ~12%, but reaches only 3-4% in higher plants and 5-7% in microalgae in empirical efficiency measurements, emphasizing the potential of algae as a resource of improvement of photosynthesis in future crops.
Algal metabolic diversity, particularly in extreme environments, represents a valuable and underexploited resource for photosynthetic improvements in plants. It has previously been shown that some extremophilic algae from deserts and Antarctic lakes possess outstanding metabolic performance compared with major crops or model algae [8-10], and these examples are just beginning to scratch the surface of the breadth of algal metabolism.
Yet, dissection of the specific molecular mechanisms underlying algal super-performance cannot be gleaned from current ‘omics technologies, but requires the development of approaches for reliable estimation of in vivo metabolic fluxes (i.e. reactions rates). In a new paper in Nature Plants, we present a newly developed microfluidics system, which in combination with rapid pulse 13CO2 labelling enables a comparative view of flux in model algae and vascular plants, disclosing key differences in their metabolic networks, and providing the first targets for synthetic biology in higher-plants.
First, we used three algae with different growth rates to test whether this novel setup can reveal what distinguishes their photosynthetic metabolism rates. These algae included the fastest growing alga on record - Chlorella ohadii, and the model alga Chlamydomonas reinhardtii, which grows 3-fold slower. Our system allowed dissection of minute differences, even in the rates of extremely fast reactions, like those belonging to the main C-fixation pathway in plants - the Calvin cycle, and downstream reactions. Second, we examined these new data in view of (1) published and new results from higher plants (like Arabidopsis or maize, as model plants for C3 and C4 photosynthesis, respectively), and (2) the carbon allocation patterns in these cells to various pools, like protein, storage carbohydrates (starch) and lipids. This integrated analysis provided the metabolic network context for the observed flux patterns and explained where algae differ from each other and from higher-plants. Finally, we pointed to specific reactions and their catalyzing enzymes as those involved in the observed flux variations, and analyzed their sequence characteristics for future mechanistic work on their distinct inter-species function and possibly, metabolic engineering.
Beyond the findings described in this work, this experimental breakthrough will enable flux estimates in multiple algae and conditions to be obtained and paves the way for leveraging algal metabolic diversity to raise targets for photosynthesis/yield improvement in future crops. It will also help to more accurately define the extent to which algae may serve as models for photosynthesis in higher-plants.
|
HFSP award information Long-Term Fellowship (LT000156/2018-L): What makes a racehorse fast? Dissecting effects of photosynthetic and metabolic performance on growth Fellow: Haim Treves |


































