Tubulin heterodimers (hereafter tubulin) are the building blocks of centriolar- and ciliary microtubules. During cilium construction, intraflagellar transport (IFT) machineries mediate the transport of ciliary building blocks of tubulin from the cytoplasmic ciliary base to the tip6-8. However, it remains poorly understood how a fraction of tubulin is selected from its large cytoplasmic pool and the mechanisms that operate to deliver these specialized tubulin to construct defined lengths of centriolar- and ciliary-microtubules.
In this work, the authors have undertaken an integrated approach to dissect the conserved function of CPAP-tubulin interaction, which represents a crucial step in understanding centriolar- and ciliary-microtubule length control. Their results support a model in which CPAP/Sas-4 through the different facets of its PN2-3 domain, defines centriolar- and ciliary-microtubule lengths. Our structural studies reveal that PN2-3 embraces a ‘necklace-like’ mode for tubulin recognition, in which CPAP via its PN2-3N targets the lumen surface of β-tubulin, and PN2-3C loop-helix motif occupies the β-tubulin microtubule outer surface (Figure. 1). This unique binding mode practically approves the formation of a high-affinity CPAP-GTP-tubulin complex and simultaneously prevents the polymerization of its bound GTP-tubulin, which is reactive, unstable, and ready for microtubule build-up once released.
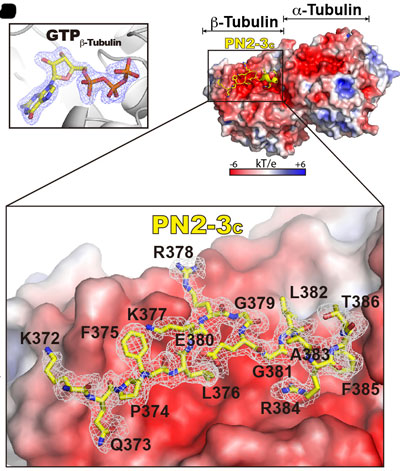
Figure 1
Surprisingly, two distinct mutations in PN2-3 exhibit opposite effects on centriolar/ciliary-microtubule lengths in vivo. CPAPF375A, with strongly reduced tubulin interaction, causes shorter centrioles and cilia exhibiting doublet- instead of triplet-microtubules. CPAPEE343RR that unmasks the β-tubulin polymerization surface displays slightly reduced tubulin-binding affinity inducing over-elongation of newly forming centriolar/ciliary microtubules by enhanced dynamic release of its bound tubulin (Figure. 2).
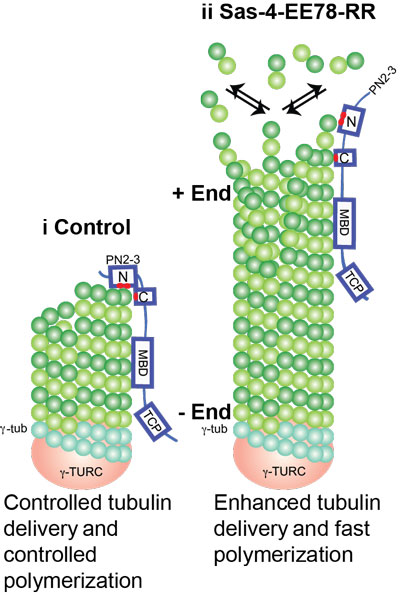
Figure 2
As free tubulin is ubiquitously present in cells, this raises the possibility of forming increased numbers and lengths of centriolar and ciliary structures. However, cells have strict mechanisms to prevent this. One such mechanism is CPAP/Sas-4-tubulin in the cytoplasm. Under physiological conditions, CPAP/Sas-4 has lower expression levels in contrast to free tubulins in cells9. Thus, at equilibrium, the amount of cytoplasmic tubulin bound by CPAP/Sas-4 making it unavailable for polymerization would only be a small fraction of the free tubulin pool. Therefore, it makes sense that CPAP/Sas-4 has a dedicated function in binding limited amounts of cytoplasmic tubulin and licenses them for centriolar- and ciliary-microtubules, which is then spatiotemporally regulated for constructing defined numbers and lengths of centriolar and ciliary structures.
Reference
Molecular basis for CPAP-tubulin interaction in controlling centriolar and ciliary length. Zheng X, Ramani A, Soni K, Gottardo M, Zheng S, Ming Gooi L, Li W2, Feng S, Mariappan A, Wason A, Widlund P, Pozniakovsky A, Poser I, Deng H, Ou G, Riparbelli M, Giuliano C, Hyman AA, Sattler M, Gopalakrishnan J, Li H. Nat Commun. 2016 Jun 16;7:11874. doi: 10.1038/ncomms11874.
Other references
1 Azimzadeh, J. & Marshall, W. F. Building the Centriole. Current biology : CB 20, R816-R825, doi:S0960-9822(10)01000-6 [pii] 10.1016/j.cub.2010.08.010 (2010).
2 Marshall, W. F. What is the function of centrioles? J Cell Biochem 100, 916-922 (2007).
3 Avidor-Reiss, T. & Gopalakrishnan, J. Building a centriole. Current opinion in cell biology, doi:10.1016/j.ceb.2012.10.016 (2012).
4 Nachury, M. V. How do cilia organize signalling cascades? Philosophical transactions of the Royal Society of London. Series B, Biological sciences 369, doi:10.1098/rstb.2013.0465 (2014).
5 Nigg, E. A. & Raff, J. W. Centrioles, centrosomes, and cilia in health and disease. Cell 139, 663-678, doi:S0092-8674(09)01362-2 [pii]10.1016/j.cell.2009.10.036 (2009).
6 Ishikawa, H. & Marshall, W. F. Ciliogenesis: building the cell's antenna. Nature reviews. Molecular cell biology 12, 222-234, doi:10.1038/nrm3085 (2011).
7 Bhogaraju, S. et al. Molecular basis of tubulin transport within the cilium by IFT74 and IFT81. Science 341, 1009-1012, doi:10.1126/science.1240985 (2013).
8 Craft, J. M., Harris, J. A., Hyman, S., Kner, P. & Lechtreck, K. F. Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. The Journal of cell biology 208, 223-237, doi:10.1083/jcb.201409036 (2015).
9 Dammermann, A., Maddox, P. S., Desai, A. & Oegema, K. SAS-4 is recruited to a dynamic structure in newly forming centrioles that is stabilized by the gamma-tubulin-mediated addition of centriolar microtubules. The Journal of cell biology 180, 771-785 (2008).


































