As the brain develops, roughly 100 billion neurons make over 100 trillion connections to send and receive information. For decades, it has been widely accepted that neuronal growth is controlled by small signalling molecules which are ‘sniffed’ out by the growing neurons, telling them which way to go, so that they can find their precise target. However, we found that neuronal growth is not only controlled by these chemical signals, but also by the physical properties of their environment. Neurons possess a sense of ‘touch’: in order to move, growing neurons exert forces on their environment and mechanically interact with it.
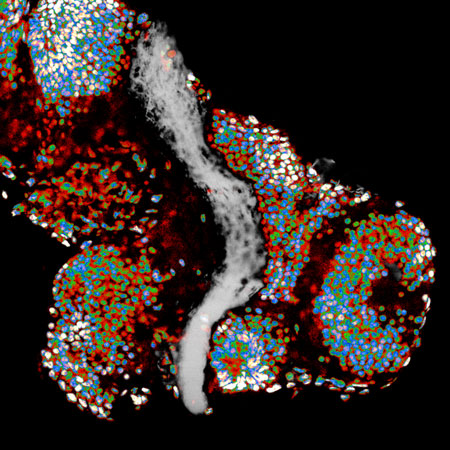
Figure: A Xenopus brain, in which cell nuclei and axons of retinal ganglion cells are labelled with false colors.
To enable our study, we developed a new technique, based on atomic force microscopy, to measure the stiffness of developing Xenopus frog brains at high resolution – revealing what axons (the long neuronal processes that relay signals to other cells) might feel as they grow through the brain. We found complex patterns of stiffness in the developing brain that seemed to predict axon growth directions. Axons avoided stiffer areas of the brain and grew towards softer regions. Changing the normal brain stiffness caused the axons to get lost and fail to find their targets.
We then explored how exactly the axons were feeling their environments. We found that neurons contain ion channels called Piezo1, which sit in the cell membrane: the barrier between cell and environment. These channels open only when a large enough force is applied, similar to shutter valves in air mattresses. Opening of these channels generates small pores in the membrane of the neurons, which allows calcium ions to enter the cells. Calcium then triggers a number of reactions that change how neurons grow.
When neuronal membranes were stiffened using a substance extracted from a spider venom, which made it harder to open the channels, neurons became ‘numb’ to environmental stiffness. This caused the axons to grow abnormally without reaching their target. Removing Piezo1 from the cells, which is another way of eliminating the axons’ capacity to feel differences in stiffness, had the same effect.
Tissue stiffness thus provides a critical signal that helps to control neuronal growth during development. It might also be involved in neuronal re-growth and regeneration. Understanding how neurons respond to mechanical signals could thus ultimately lead to the development of new strategies for promoting neuronal regeneration after, for example, spinal cord injuries.
Investigating the role of mechanics in the development of the central nervous system (CNS) and developing novel technologies to achieve this is at the core of our HFSP grant. Atomic force microscopy used here enabled first insights into in vivo brain mechanics. Exploiting the techniques developed by our team, we will soon be able to study CNS mechanics in even much greater detail.
Reference
Mechanosensing is critical for axon growth in the developing brain. Koser DE, Thompson AJ, Foster SK, Dwivedy A, Pillai EK, Sheridan GK, Svoboda H, Viana M, Costa LdF, Guck J, Holt CE, Franze K. Nature Neuroscience 19(12):1592-1598 (2016).


































