The forces exerted by cells vary substantially in magnitude, spatial distribution and temporal evolution. In particular, there is rapidly increasing interest in the role played by vertical forces, i.e. forces that cells exert perpendicularly to their substrate, e.g. during the extension of special cellular processes such as podosomes or during amoeboid migration in spatial confinement where non-specific cell-substrate interactions such as friction and pushing are important. These forces are often weak, and existing methods have struggled to resolve them in a non-disruptive manner and with sufficient throughput.
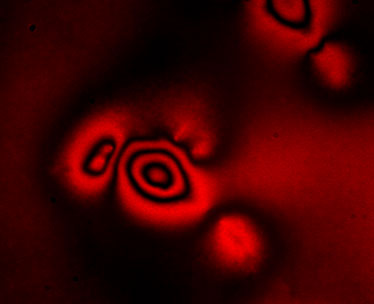
Figure: ERISM is a new microscopy method that analyses interference patterns to create images of the forces living cells apply as they grow, divide and migrate. Shown is the interference pattern generated by a human embryonic kidney cell.
We addressed this need by developing Elastic Resonator Interference Stress Microscopy (ERISM), an approach for direct, robust and non-destructive imaging of forces associated with various types of mechanical cell-substrate interactions. While most existing methods use localization microscopy or direct imaging of surface deformations, ERISM is inspired by the beautiful rainbows of colours on the surface of soap bubbles. Using the optical principle of thin-film interference, allowed us to detect cell-induced substrate deformations with unprecedented sensitivity. With this, we are not only able to resolve forces exerted by cells that form firm focal adhesion contacts to the substrate but can also detect protein-specific cell-substrate interaction and quantify the much weaker vertical forces (down to piconewtons) associated with amoeboid-type cell migration through confined environments and with the protrusion of podosomes. ERISM requires no zero-force reference image, which eliminates the need to detach non-migrating cells after a measurement and enables continuous, long-term measurements of multiple cells on one substrate as well as further investigation of the cells, e.g. by immunostaining. Being a wide-field imaging method, ERISM determines the local deformation at each point of the image simultaneously and requires only low light intensities, thus facilitating observation of multiple cells at once without inducing photo-damage to the cells. This robustness means that measuring cell forces could soon become a tool in clinical diagnostics, through which doctors could complement existing techniques to assess the invasiveness of cancer.
The development of ERISM is one component of a broader project of a recently completed HFSP Young Investigator Grant that integrates expertise in optics, biophysics and neurobiology to investigate the role of mechanics in the development of the central nervous system (CNS). CNS development is one of the most spectacular processes in biology. Key aspects include the formation of neuronal axons, their subsequent growth and guidance through thick layers of nerve tissue, and the folding of the brain. All these processes involve motion and are driven by forces. However, while our understanding of the biochemical and molecular control of these processes is increasing rapidly, the contribution of the dynamic interplay between cellular forces and tissue elasticity remains poorly understood – mostly due to a lack of suitable measurement techniques.
To address this need, a novel photonic toolbox was developed in this project for in situ, label-free and non-contact measurements of cellular forces and elasticity. Force sensing (developed in the lab of Dr. Malte Gather at the University of St Andrews, UK) is based on the ERISM method described here. Elasticity measurements (developed in the lab of Dr. Scarcelli at the University of Maryland, USA) is based on high-resolution Brillouin microscopy. Force and elasticity measurements are combined to illuminate how forces exerted by neurons contribute to axon formation and neuronal guidance (led by the lab of Dr. Kristian Franze at the University of Cambridge UK).
Reference
Long-Term Imaging of Cellular Forces with High Precision by Elastic Resonator Interference Stress Microscopy. NM Kronenberg, P Liehm, A Steude, JA Knipper, JG Borger, G Scarcelli, K Franze, SJ Powis, MC Gather, Nature Cell Biology 19, 864–872 (2017).


































