The basal ganglia are a collection of brain centers that guide motivated actions and habitual behaviors. The neurochemical dopamine motivates and invigorates actions through its release in the striatum. The striatum has the highest brain expression of dopamine and also another neurochemical, acetylcholine. Indeed, one of the oldest ideas in striatal physiology is that a balance needs to be maintained between the levels of dopamine and acetylcholine in the striatum for the basal ganglia to function normally. Various neurological disorders are associated with a disruption of this balance. In Parkinson’s disease, there is a reduction in striatal dopamine and an increase in striatal acetylcholine. Conversely, in the early stages of Huntington’s disease, there is a reduction in acetylcholine and an elevation of dopamine. However, we do not know whether this dopamine-acetylcholine balance is maintained or indeed is required during the normal function of striatal circuitry and, if so, how it is realized.
In a study published in 2021 by researchers at Brown University (Hamid et al. Cell 2021), Dr. Arif Hamid and co-workers used leading-edge techniques to visualize striatal dopamine release in real-time in behaving mice and reported that the release takes the form of waves of dopamine release that travel through the striatum. They also provided evidence that the direction of the waves depends on the kind of behavior the animals were engaged in. The HFSP Research Grant Awardees team used comparable techniques that enable the visualization of acetylcholine release in real-time and found that acetylcholine is also released in the form of waves that travel through the striatum in similar directions.
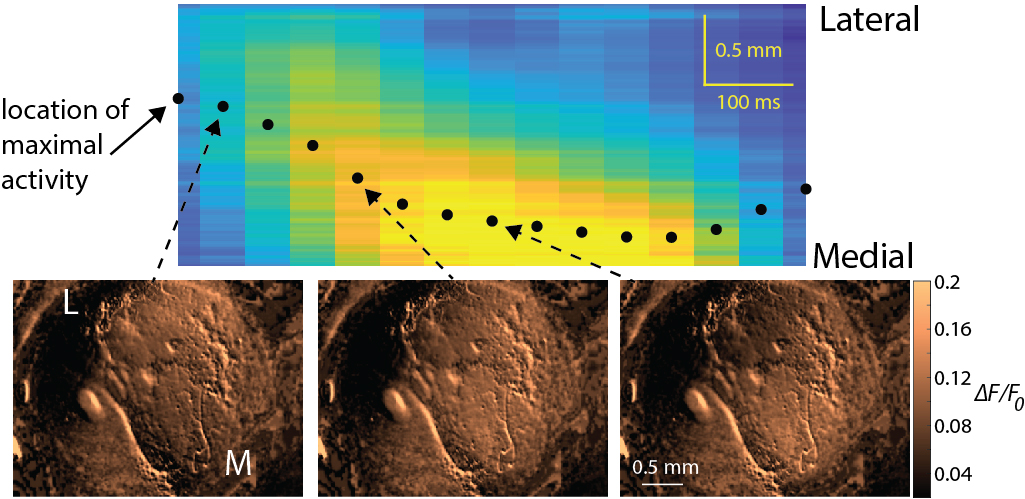
Finding the acetylcholine waves led the researchers to speculate whether the two types of striatal neurochemical waves could be related. Furthermore, inspired by the dopamine–acetylcholine balance hypothesis, the team asked whether there could be a joint mechanism that could give rise simultaneously to dopamine and acetylcholine waves. To address these questions, the scientists turned to mathematical modeling. They realized that the known local interaction between acetylcholine and dopamine is reminiscent of a non-linear activator inhibitor chemical interaction. Additionally, the Research Grant Awardees hypothesized that the dense space-filling network of dopamine- and acetylcholine-releasing axons acts like two coupled extended diffusive media (of electrical activity). Thus, using mathematical modeling, they were able to reduce the known interaction between dopamine axons and acetylcholine-releasing – or cholinergic – interneurons in the striatal microcircuitry to an activator-inhibitor reaction-diffusion (AIRD) system. AIRD systems are famous for giving rise to traveling wave solutions. In the study, published recently in the scientific journal Nature Communications, the HFSP awardees considered various parameter regimes of the AIRD systems and reduced this system to simpler tractable systems (which maintain the same phase-plane geometry) to analyze their phase diagrams and show how they could give rise to empirically measured correlations (Howe et al. eLife 2019) between striatal dopamine and acetylcholine.
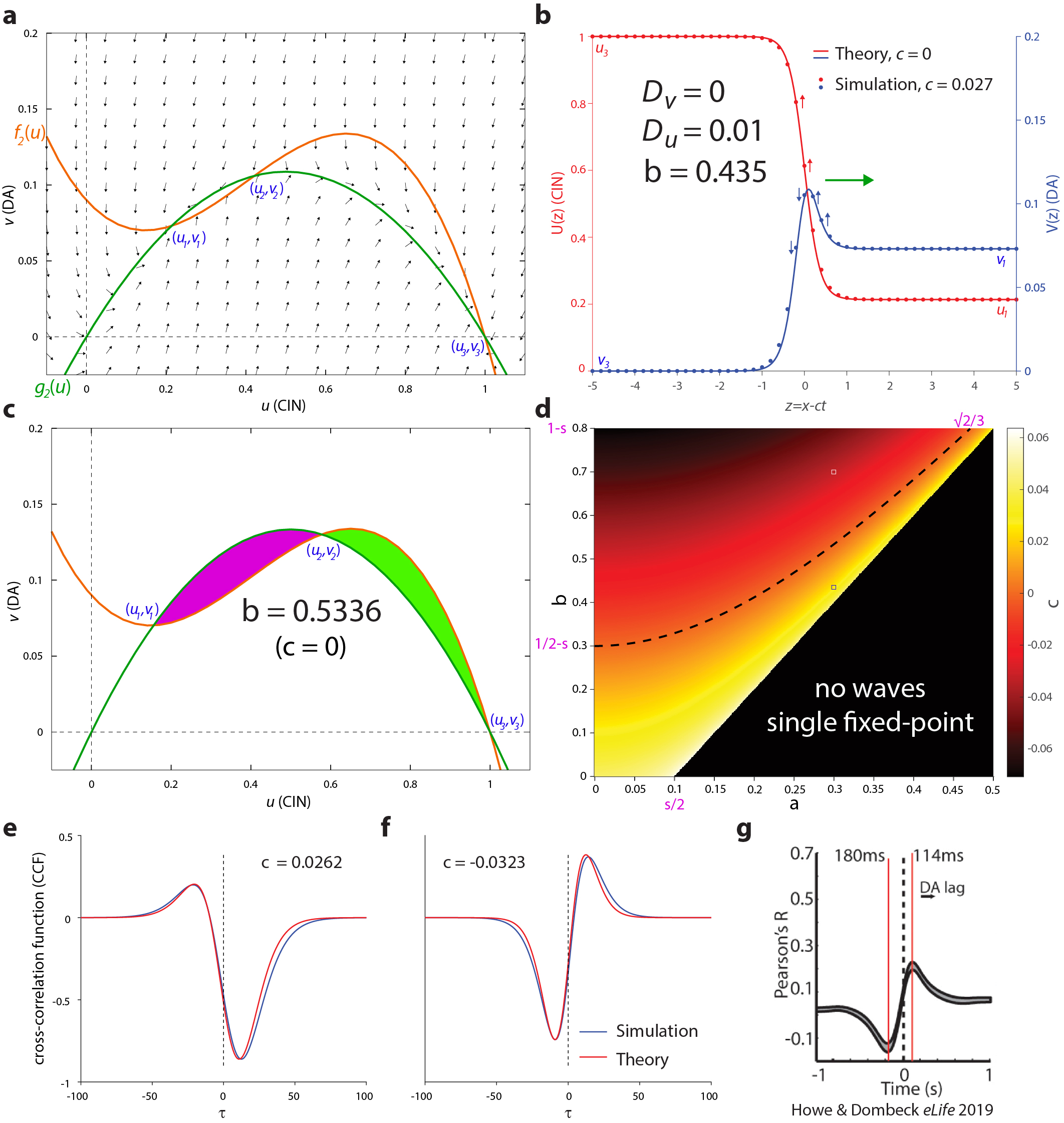
The analysis also reveals regions of the phase diagram where Turing patterns can form in the wake of traveling waves. Importantly, the models strongly predict that if dopamine and acetylcholine waves would be imaged simultaneously, they would share a wavefront. They also predict that the formation and direction of travel of the waves should depend on the activity of various interneurons in the striatal circuitry.
Some of the model assumptions were also tested empirically. One of these was the assumption that localized acetylcholine release, even by a single cholinergic interneuron, can induce nearby localized dopamine release.
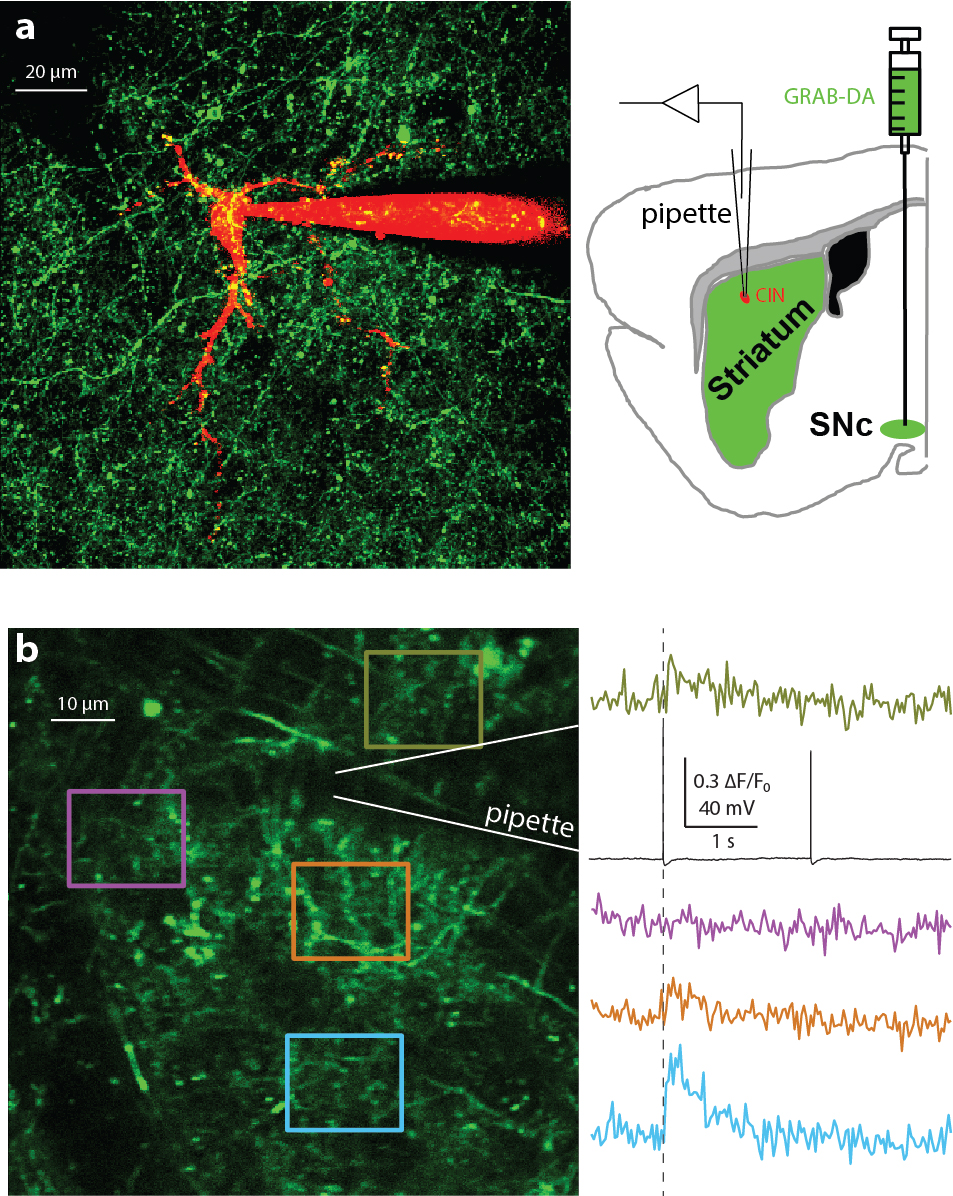
The scientists were able to use electrophysiological techniques in conjunction with multiphoton imaging to demonstrate this finding, which overturned previous studies that argued that only synchronous activation of several cholinergic interneurons can do this (Threlfell et al. Neuron 2012; Cachope et al. Cell Rep 2012; Kramer et al. Neuron 2022). In summary, the study provided novel evidence of acetylcholine waves and proposed a mathematical theory for how a balance or interaction between striatal acetylcholine and dopamine could be realized through the formation of joint traveling waves.
|
|
|
|
Du/Dv=0.01 |
|
|
|
|
|
Du/Dv =0.015 |
|
|
|
|
|
Du/Dv =0.02 |
|
|
Stable and unstable Turing patterns forming in the wake of a traveling wave in model. red: acetylcholine; blue: dopamine. |
|
In 2018. Dr. Josh Goldberg (Hebrew University of Jerusalem) attended the Basal Ganglia Gordon Research Conference, where he saw Dr. Hamid (Brown University) presenting his findings on dopamine waves. Having seen indications of acetylcholine waves in his lab, Dr. Goldberg approached Dr. Jeff Wickens (Okinawa Institute of Science and Technology Graduate University), who was among the scientists who reported that dopamine dynamics in the striatum might arise locally in the striatum (i.e., diverge from the spiking activity of the dopamine cell bodies in the midbrain). At that meeting, they brainstormed about the possibility that the same mechanism generates dopamine and the putative acetylcholine waves. Dr. Wickens suggested collaborating on this project and submitting an application for an HFSP grant. He also suggested (again, at the same meeting) to recruit Dr. Lin Tian (UC Davis) because she develops new genetically encoded indicators of various neurochemicals that could be used in the planned experiments. In 2019, the team was awarded an HFSP grant that funded this project and culminated in the present publication.
Note: all images/figures belong to the research authors.