In 2000, Jean-Luc Popot was awarded an HFSP Research Grant, together with his colleagues Edward A. Berry and Catherine Vénien-Bryan, to develop synthetic polymers called "amphipols", which they hoped would provide membrane biologists with new tools to handle membrane proteins in aqueous solutions. As briefly recounted below, this help proved absolutely decisive as the project was too novel, basic and risky to be fundable for a sufficient duration at the French or the European level.
| Jean-Luc Popot, an HFSP Research Grant alumnus, founded and directed the Laboratoire de Biologie Physico-Chimique des Protéines Membranaires at the Institut de Biologie Physico-Chimique in Paris, France. His background is in biochemistry and biophysics, with a special interest in physical chemistry. His work has focused on integral membrane proteins, with a bend towards developing general methods or concepts, applicable to most if not all integral membrane proteins, and, in particular, understanding the mechanisms that determine their folding, assembly and stability. He was especially interested in those membrane proteins whose transmembrane domain is made up of α-helices. In his work he also explored new methods of sequence analysis and developed novel chemical tools for handling and/or studying membrane proteins, in particular two families of surfactants, fluorinated surfactants and amphiphilic polymers ('amphipols'). His latest work has mainly focused on developing amphipols and their applications. |
Membrane proteins (MPs) are key players in biology because of their role, among others, in ensuring exchanges between and within cells, energy transduction and monitoring of and response to environmental changes. Indeed, more than 60% of current drugs target MPs. Much information about how MPs function can be obtained in vivo using electrophysiology, imaging, monitoring of physiological responses, etc. However, a detailed understanding of the structure of MPs, how they accomplish their tasks and how their function can be modulated by drugs requires, sooner or later, that they be extracted from their original membrane environment and handled in aqueous solutions. Because the transmembrane surface of MPs is highly hydrophobic, this cannot be achieved without replacing the membrane with another surfactant. Traditionally, this surfactant is a detergent, which disrupts the membrane, disperses its components, and, by adsorbing onto the transmembrane surface of the MP, makes it hydrophilic and keeps the protein from precipitating.
Detergents, however, are dissociating, aggressive molecules, and the life expectancy of a detergent-solubilized MP is usually short. This has led chemists and biochemists to try to develop so-called "mild" detergents, as well as alternative surfactants that present little or no detergency and, as a rule, are substituted for detergents after the solubilization step.
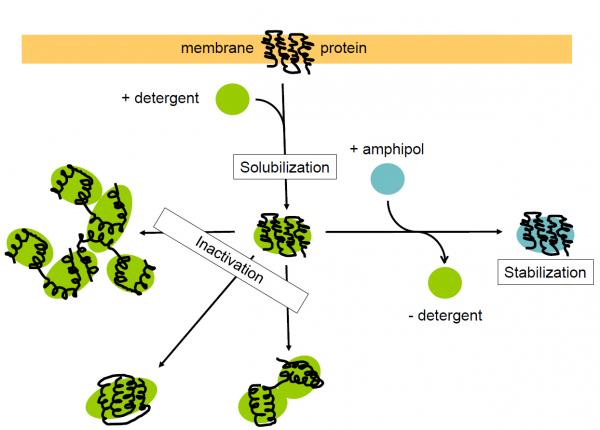
Figure 1: Trapping and stabilizing a membrane protein with amphipols: a schematic representation of what is happening at the molecular level and the consequences on the protein.
In 1996, we introduced short amphipathic synthetic polymers, which we had dubbed "amphipols" (APols), designed as a substitute for detergents to keep MPs water-soluble 1. It turned out that APols, as we had hoped, stabilize most MPs compared to detergent solutions 2. Understanding and tailoring the properties of APols and developing their applications is a protracted endeavor that started in 1994 and continues today (for recent reviews, see refs. 3, 4 & 5). Whereas APols are now on their way to becoming routine tools for such applications as folding MPs obtained as inclusion bodies, imaging them by electron microscopy, or studying them by solution NMR, such was not the case in the '90s, when we were groping our way more or less in the dark, trying to identify both pitfalls and opportunities. The project was initially funded by an interdisciplinary grant from the French Centre National de la Recherche Scientifique (CNRS), then by a European grant, but these sources of funding quickly ran dry. In France, the CNRS was progressively deprived of the means to fund its own research projects. Any remaining research funds were being diverted either to industry or to an agency under direct control of the Ministry, with, as could be expected, a strong bend towards targeted, short-term research. At the European level, funding tends to go to very large projects managed by big consortia, which generally run along predictable roads ("take 100 proteins, apply to each 10 different approaches, hire 20 postdocs and crank the handle, some results are bound to follow"; yes, but which, and at what cost?). Chances for small labs to be funded to carry out risky, purely basic research are almost nonexistent.
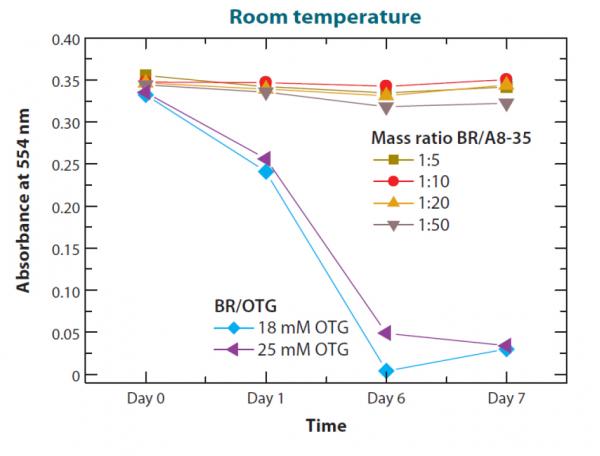
Figure 2: Illustration of the stabilizing effect of amphipols on the model protein bacteriorhodopsin (BR). A8-35 is an amphipol, OTG, a detergent. Source: ref. 4
By the end of the '90s, we had only managed to publish four articles, one more was in the making, and we were hard put to come up with the means to net and land what we felt was a pretty big but combative fish. In despair, we applied for an HFSP grant to develop APols for MP structural studies and, much to our surprise, we were awarded it. The committee apparently had perceived that the project, albeit purely basic and a long shot, could, if successful, yield important dividends in many areas of membrane biology and it daringly bet on it. This was the decisive impetus that brought us above the free-energy barrier blocking us from developing this novel technology. Over the following three years, we were free to sort out the sources of the vagaries that had plagued some of our early experiments and move forward. By the end of the grant, seven more articles had been published, the source of the difficulties we had met during some syntheses had been identified and brought under control, and a network of international collaborations had been built, which would, in the following years, result in a flourishing of applications. Some of those had been more or less predictable from the start, whereas others – such as using APols to fold MPs – were unanticipated bonuses.
By 2005, a sufficiently solid platform of data had been established, enabling us to compete with big consortia on the European grant market and participate in a successful NIH grant application. As of today, close to 200 articles involving APols have been published at a rate that keeps steadily increasing: in 2014, an especially rich year, it topped 40 publications. The highest resolution single-particle EM images of MPs ever obtained, which for the first time were detailed enough to permit the direct building of atomic models into electron density maps, were achieved - along with major technical advances - thanks to the use of APols 6; 7. Applications have expanded well beyond structural biology and even beyond basic research, with therapeutic uses starting to be explored, e.g., for formulating MP-based vaccines 8; 9. What started in 1994 as a very risky bet has matured into a well-developed methodology, bringing a modest but useful contribution to the toolbox of membrane biologists.
Before concluding, let me say a word in praise of HFSP's administration. Writing the grant application was a serious piece of work, almost on a par with writing up a European one, although with less nonscientific padding. After the grant had been awarded, however, in strong contrast to other funding organizations, the demands of HFSP regarding financial and scientific reports were kept to a minimum until the end of the project, allowing us to fully concentrate on the science and not divert any time or resources to administrative paperwork.
Finally, I would like to come back to the decisive role that HFSP played in this story. There is no doubt in my mind that, without their timely help, the project would have withered and finally died from lack of support, and that no membrane biologist would use APols today. Thanks to HFSP, we succeeded, but how many equally good or better projects are disregarded by review panels for want of being big enough or their outcome predictable enough? As is frequently recalled, but rarely heeded, it is not by improving the candle that one is going to invent LEDs: it takes decades of non-targeted fundamental research. It is vital to the future of research that risky, imaginative, unconventional projects get funded, and HFSP ought to be commended for being willing to take such a risk, something that not many funding agencies do.
|
References 1. Tribet, C., Audebert, R. & Popot, J.-L. (1996). Amphipols: polymers that keep membrane proteins soluble in aqueous solutions. Proc. Natl. Acad. Sci. USA 93, 15047-15050. 2. Kleinschmidt, J. H. & Popot, J.-L. (2014). Folding and stability of integral membrane proteins in amphipols. Arch. Biochem. Biophys. 564, 327-343. 3. Popot, J.-L. (2010). Amphipols, nanodiscs, and fluorinated surfactants: Three non-conventional approaches to studying membrane proteins in aqueous solutions. Annu. Rev. Biochem. 79, 737-775. 4. Popot, J.-L., Althoff, T., Bagnard, D., Banères, J.-L., Bazzacco, P., Billon-Denis, E., Catoire, L. J., Champeil, P., Charvolin, D., Cocco, M. J., Crémel, G., Dahmane, T., de la Maza, L. M., Ebel, C., Gabel, F., Giusti, F., Gohon, Y., Goormaghtigh, E., Guittet, E., Kleinschmidt, J. H., Kühlbrandt, W., Le Bon, C., Martinez, K. L., Picard, M., Pucci, B., Rappaport, F., Sachs, J. N., Tribet, C., van Heijenoort, C., Wien, F., Zito, F. & Zoonens, M. (2011). Amphipols from A to Z. Annu. Rev. Biophys. 40, 379-408. 5. Zoonens, M. & Popot, J.-L. (2014). Amphipols for each season. J. Membr. Biol. 247, 759-796. 6. Liao, M., Cao, E., Julius, D. & Cheng, Y. (2013). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107-112. 7. Lu, P., Bai, X.-c., Ma, D., Xie, T., Yan, C., Sun, L., Yang, G., Zhao, Y., Zhou, R., Scheres, S. H. W. & Shi, Y. (2014). Three-dimensional structure of human γ-secretase. Nature 512, 166-170. 8. Tifrea, D. F., Sun, G., Pal, S., Zardeneta, G., Cocco, M. J., Popot, J.-L. & de la Maza, L. M. (2011). Amphipols stabilize the Chlamydia major outer membrane protein and enhance its protective ability as a vaccine. Vaccine 29, 4623-4631. 9. Tifrea, D., Pal, S., Cocco, M. J., Popot, J.-L. & de la Maza, L. M. (2014). Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with C. muridarum. J. Immunol. 192, 5201-5213. |


































