An ongoing challenge for scientists working to understand the brain is being able to see all its parts. Researchers have spent centuries developing better imaging techniques to see beyond the ability of the naked eye. They’ve built microscopes that gather information down to the level of the electron. They’ve engineered fluorescent tags that make cells and structures of interest more visible. One of the most effective imaging techniques for neuroscientists has been the combination of FRET (Fluorescence Resonance Energy Transfer) and FLIM (Fluorescence Lifetime Imaging Microscopy). This duo gives scientists the power to view biochemical dynamics of proteins with high spatial and temporal accuracy, while also allowing them to calculate the miniscule distances between molecules in real time.
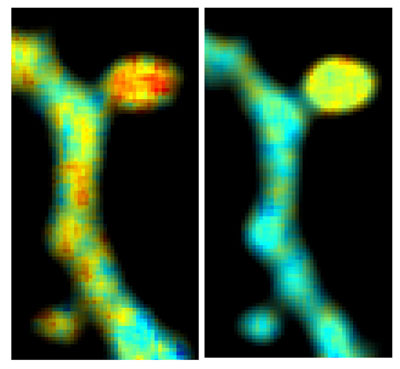
Figure: Dual color FLIM image showing differential activation levels of two signaling molecules, RhoA (left) and CaMKII (right), in a dendritic spine undergoing structural plasticity. Color scale represents difference in activity levels (red - high, blue - low).
At the Max Planck Florida Institute for Neuroscience (MPFI), researchers in Ryohei Yasuda’s laboratory, including HFSP Long-Term Fellow, Tal Laviv, have been working to understand the cellular and molecular mechanisms of learning and memory. The team has been using FRET-FLIM to study the activity of proteins in dendritic spines, protrusions that form off neuronal branches, and make synaptic connections and communicate with other neurons. Dendritic spines are known to emerge, change shape, and even disappear over a lifetime, and these changes are considered the cellular basis for learning and memory. These imaging techniques were a key factor in helping the team elucidate some of the molecular mechanisms behind this type of plasticity.
Nonetheless, there is an important limitation in using these techniques to understand how multiple types of proteins and molecules interact in living samples. There is only one FRET donor tag (GFP) that will work within a FLIM protocol, so if researchers want to study two proteins, they’ll investigate “Protein A” in one set of experiments, following with an additional set of experiments to examine “Protein B” activity. Following these experiments, the researchers will need to draw conclusions about how these two proteins interact based on the two sets of experiments.
Researchers at Stanford University, from the team of Dr. Michael Lin, reached out to the Yasuda Lab at MPFI after they identified a new set of red fluorescent proteins, or RFPs, named CyRFPs. They suggested this new set of RFPs could be used in combination with GFP for simultaneous imaging.
To determine if this could work, the scientists tested the ability to visualize dendritic spine structure and function using CyRFP and a GFP based calcium bio-sensor. With this combination, they were able to monitor the structure and function of spines in real time, even in the brains of living animals. Finally, the team tweaked a variant of CyRFP, which now could be used as a fluorescent FRET donor as a part of a FRET pair, named monomeric cyan-excitable red fluorescent protein (mCyRFP1). Scientists in the Yasuda Lab conducted a series of experiments to test the newly proposed FRET pair alongside a GFP biosensor. The technique allowed them to view, for the first time, the activities of two signalling molecules within a single dendritic spine as the spine was undergoing synaptic plasticity.
Dr. Laviv explains that the new technique will increase both accuracy and efficiency of FRET-FLIM imaging experiments and could potentially increase our understanding of how learning and memory ultimately alter the structure and function of dendritic spines.
Reference
Simultaneous dual-color fluorescence lifetime imaging with novel red-shifted fluorescent proteins. Tal Laviv, Benjamin B. Kim, Jun Chu, Amy J. Lam, Michael Z. Lin, Ryohei Yasuda. Nature Methods, doi:10.1038/nmeth.4046


































