The nuclear envelope serves as a barrier between the nucleus and cytoplasm, which was normally presumed to breakdown only during a controlled process of cell division. It is important for the cell to maintain this barrier between the nucleus and cytoplasm because there are proteins and molecular components that should be kept inside the nucleus or conversely for others to be kept outside the nucleus for the cell to function properly. And while it is perhaps not too surprising that applying an external mechanic stress on the nucleus may rupture this nuclear envelope barrier, the concept that the cell can induce this rupture on itself from migrating through a small pore is less expected. Moreover, how exactly the nuclear envelope reseals is not known.
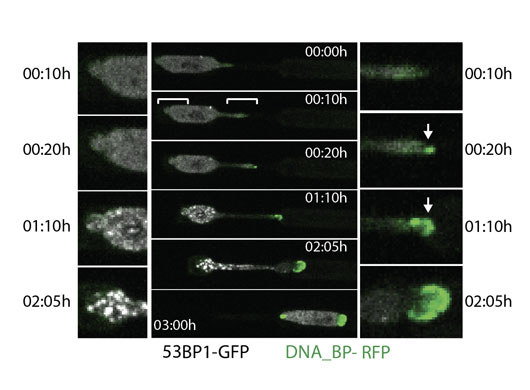
Figure: A nucleus stretches as it moves through a constriction. It makes a dumbbell shape as it is moving in the rightward direction. The front tip of the nucleus on the right side marks the entry of a RFP tagged DNA binding protein (green), which indicates opening of the nuclear envelope on this side. 53BP1 tagged with GFP (white) indicates sites of DNA repair as discrete foci within the nucleus. Time is hours:min. Scale bar 10µm.
We found that fast moving immune cells migrating inside tissue explants, but also slow moving cancer cells, indeed open their nucleus, by fluorescent tracking of a nuclear localized signal (NLS), which leaked into the cytoplasm when the nucleus deformed. When cells migrated through micro-channels constructed with narrow tubular constrictions which serve to force the nucleus to constrict when migrating through, we were able to increase or decrease the amount of nuclear rupture that occurred. The narrower the constriction of the nucleus, the higher the amount of nuclear opening that occurred. This rupture was attributed to a pressure difference between the nucleus and the cytoplasm because the initial site of the location of the opening was the nuclear tip, determined with a fluorescently tagged DNA binding protein, as expected from the Laplace law. The opening of the nuclear envelope was transient and the kinetics of the resealing were measured to be a couple of minutes. The restoration of the integrity of the nucleo-cytoplasmic barrier was assessed by reentry of fluorescently tagged NLS. Moreover, this nuclear resealing was mediated by membrane remodeling complexes (ESCRT), which have a variety of functions serving to fuse membranes together inside the cell.
The opening of the nuclear envelope also coincided with DNA damage, assessed by the accumulation of a DNA repair factor, GFP tagged 53BP1, on foci corresponding to DNA breaks. When the DNA damage response was inhibited with a drug, thus handicapping the repair of the DNA, cells rupturing their nucleus were more likely to die. Thus, cells migrating through small pores rupture their nuclear envelope, leading to DNA damage which is normally repaired efficiently, but cell death occurs when the cell is rendered unable to repair the DNA. The impact of this study can be extended to normal homeostatic functions involving cell migration in tissues, as nuclear envelope opening may be affecting cell function.
Reference
ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Raab M, Gentili M, de Belly H, Thiam HR, Vargas P, Jimenez AJ, Lautenschlaeger F, Voituriez R, Lennon-Duménil AM, Manel N, Piel M. Science. 2016 Apr 15;352(6283):359-62. doi: 10.1126/science.aad7611.


































