Synapses form functional units by segregating and condensing component proteins beneath the synaptic contact. Such structures are necessary for the regulation of synaptic transmission and plasticity. Interestingly, the condensate lacks demarcating membrane and the components are constantly exchanged with the surrounding environment but it can still stably maintain its components and structure. Nevertheless, a neuronal activation affects the stability and enrichment of the protein condensate at the synapse. How a neuron accomplishes such regulation remained largely elusive.
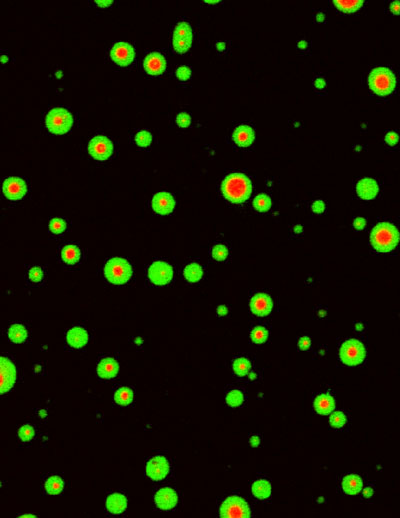
Figure 1: Liquid-liquid phase separation of CaMKII: activation of CaMKII induces segregation of NMDA receptors (green) and AMPA receptors (red).
Liquid-liquid phase separation (LLPS) is a phenomena where biological macromolecules form spontaneous condensate. Emerging evidence suggests that those condensates participate in various cellular functions through the formation of membrane-less organelles. Previous studies revealed the involvement of LLPS of pre- or post-synaptic proteins in synapse function. Postsynaptic density (PSD), a protein condensate beneath the postsynaptic membrane regulating precise localization and stability of transmitter receptors, also formed via LLPS. But how PSD responds to calcium ions – the main triggers of memory formation – has not been revealed. To this end, we aimed to reconstitute calcium-dependent synaptic memory via LLPS with a minimum number of purified PSD proteins.
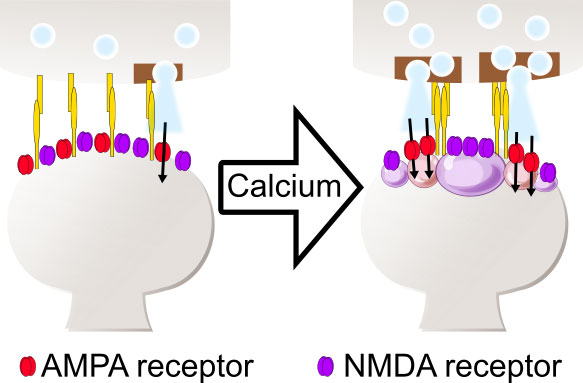
Figure 2: A novel mechanism of synaptic plasticity: formation of LLPS protein condensates as a segregated nanodomain between AMPA receptors and NMDA receptors enhances the efficacy of synaptic transmission as a novel mechanism of synaptic plasticity.
As a model of synaptic memory, we focused on a phenomenon called long-term potentiation (LTP). LTP is triggered by an influx of calcium ions via the cell surface channel. Thus, we hypothesized that there might be a calcium-responsible factor in PSD that induces LLPS. We deemed calcium/calmodulin-dependent protein kinase II (CaMKII) may play such a role. Indeed, CaMKII is widely known as a regulator of synaptic strength. The activation of CaMKII by calcium influx results in the phosphorylation of PSD proteins and the enlargement of the postsynaptic structure. However, CaMKII is also known as a highly abundant protein in neurons, especially in PSD. In addition, CaMKII forms a dodecameric complex, which is not necessary for its enzymatic activity. This multimeric structure and abundance is ideal to undergo LLPS.
Indeed, we found that CaMKII undergoes LLPS and forms a macromolecular complex with NMDA type glutamate receptors when activated by calcium. Interestingly, even after removal of the calcium, CaMKII condensate sustained via autophosphorylation. We also carried out a similar experiment in the presence of an AMPA type glutamate receptor, in addition to the NMDA receptor. We found that the activation of CaMKII resulted in the formation of AMPA receptor condensation inside of the NMDA receptor condensation, thereby making phase-in-phase structure. Through this mechanism, calcium can segregate two types of glutamate receptors, NMDA and AMPA receptors.
The recent development of super resolution microscopy revealed that AMPA and NMDA receptors are forming discrete clusters at the synapse, named nanodomains. Nanodomains align the presynaptic transmitter release site through adhesion molecules to enhance the efficacy of synaptic transmission, named as a nanocolumn. The formation of nanocolumns is regulated by synaptic activity, thereby explaining synaptic plasticity. We wondered if the phase-in-phase structure we found in purified proteins is the underlying mechanism of the formation of nanocolumns. We employed super-resolution microscopy to observe NMDA and AMPA receptors at the synapse. In naïve synapses, the NMDA and AMPA receptors are segregated from each other. But disruption of the CaMKII-NMDA receptor resulted in a reduction in segregation. These results are consistent with the results of the LLPS experiment.
Taken together, we found the LLPS of CaMKII triggered by calcium is a novel mechanism of synaptic memory.


































