Cells migrate actively inside our bodies during normal conditions, for example during development, tissue repair and immune patrolling, but also in disease such as metastasis during cancer progression. Regardless of the cell type, motile cells making their way inside living tissues often encounter obstacles and junctions, where their path branches into alternative directions of migration. This is the case of cells moving along blood vessels, which often bifurcate into branches to irrigate different locations.
There are different migratory strategies that differentially rely on the adhesion to the substrate. Cells that undergo mesenchymal migration require the adhesion to the substrate, and also the ability to detach from it, just like a gecko that is vertically climbing a tree or walking upside down on a branch. Similar to the gecko, cells undergo stick-slip motion, which is the alternation of slow and fast motility phases as the cell modifies their stickiness to the surface. During the slow motility phases, cells elongate thanks to cytoskeleton-mediated membrane protrusions and new focal adhesions at the cell front. On the other hand, the fast motility phases are characterized by the retraction of the tail due to the detachment of the old focal adhesions at the cell rear. This migration can be sustained in one direction as long as the cell maintains its polarity axis, but what happens if cells face a bifurcation and needs to choose a new direction?
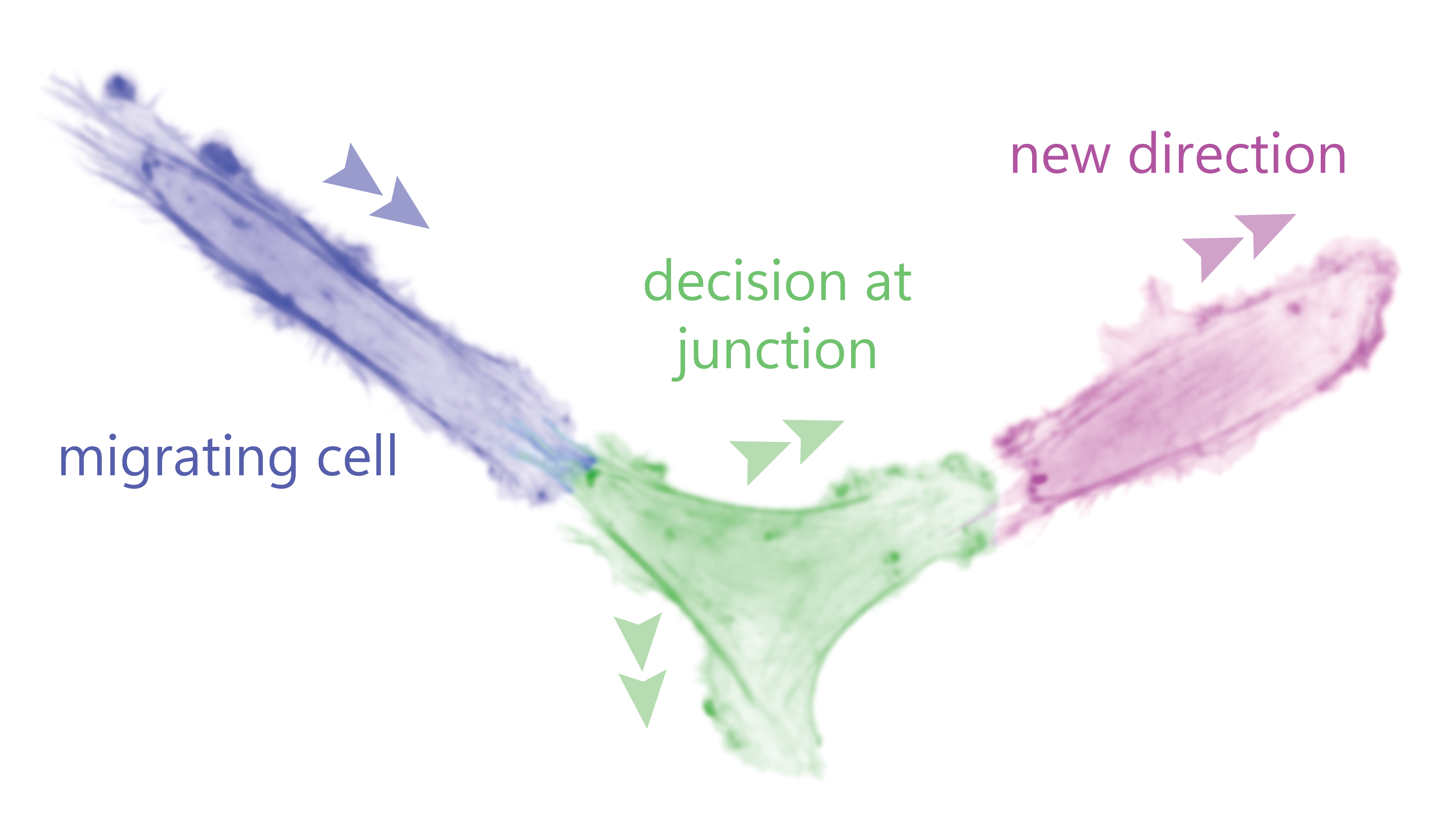
In a recently published study (Ron et al.), the HFSP team members Nir S. Gov (Weizmann institute, Israel) and Pablo J. Sáez (UKE, Germany), and an international collaborator Nils Gauthier (IFOM, Italy) analyzed how cancerous and non-cancerous (endothelial) cells behave when facing a junction that forces them to decide a new direction. Using live-cell imaging, advanced image analysis, micropatterns, and a new theoretical model the researchers predicted and experimentally demonstrated the spontaneous emergence of out of phase membrane oscillations in the competing arms before the new direction is chosen. This response depended on how cells polarize their cytoskeleton, particularly actin, while moving along one-dimensional lines that end up in a symmetric Y-junction. At the junction the cells have 4 possible outcomes: choosing one of the 2 arms in front, turn back from where it came, or getting stuck as there is no retraction and the 3 competing arms remain active. Most cells take a decision and continue their migration forward by choosing one of the paths explored by the 2 competing arms at the cell front. We have named this process directional decision-making.
Using this new theoretical model and experimental validation, the team also found that cancer cells spend a significantly shorter time at the junction before making a decision. These results could be explained by increased invasive capacity exhibited by cancer cells, which leads to tumor progression. The theoretical model could also be expanded to similar scenarios for other cells, such as leukocytes, which undergo amoeboid migration. This research therefore exposes a novel phenomenon of how cells migrate in a complex geometry with different unbiased directional options, which is fundamental for numerous biological processes.


































