Malaria causing parasites are transmitted by mosquitoes into our skin. In the skin parasites migrate to find a blood capillary into which they enter to continue the arduous journey along their complex life cycle. The parasites move 10 times faster than the phagocytes of our immune system, hence literally outrunning our innate defences. This rapid motility might thus be one of the Achilles heals of the parasite. It is, for sure, a fascinating process to study as the cells move at this high speed without changing their shape. Like in our cells, including phagocytes, the motility of the parasite is based on an actin-myosin motor. Yet, there are both striking and subtle differences in the way the parasites organize this motor and translate the force into movement. During the long divergent evolution of the parasite, it has come to rely on short and highly dynamic actin filaments. This is in stark contrast to our cells, which can build long filaments and arrange them into impressive bundles. In the parasite, myosin appears to push the actin filaments backwards, which leads to the flow of surface proteins from the front to the rear. When these surface proteins are coupled to the substrate, motility ensues.
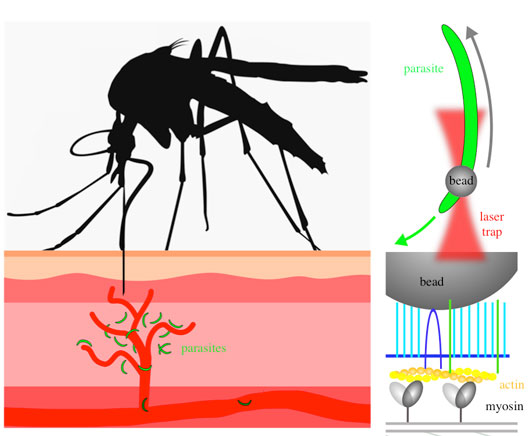
Figure - Left: a mosquito (black) injects Plasmodium parasites (green) into the skin. The parasites move rapidly at speeds of 1-2 micrometer per second to enter into blood capillaries (red).
Right, top: An isolated parasite (green) moves along the direction indicated by the green arrow. A small bead is placed with a laser trap (red) onto the front end of the parasite and is moved rearwards along the parasite surface by the actin-myosin motor of the parasite (grey arrow).
Right, bottom: The bead is bound to the parasite surface via a number of proteins that span the plasma membrane (blue). Underneath the plasma membrane the actin myosin motor is anchored in a parasite specific organelle. Movements of myosin (indicated through shading) translocate actin filaments (yellow/orange), which in turn move the bead. Note that the generated force is about 10.000 billion fold smaller than the force exerted by a standing human onto the ground.
In order to understand how the parasite achieves its high speeds we study actin itself, actin binding proteins and proteins linking the motor across the plasma membrane to the substrate using a range of microscopy and biophysical methods. To understand the forces generated by the parasite we used optical tweezers to place microscopic beads onto the front part of motile parasites. Surprisingly the beads were moved rearwards much faster than the parasites moved forward themselves. The speed was even increased when one of several surface proteins called TLP involved in motility was deleted. Force measurements on the beads indicated that the parasites generate about 100 pN of force, suggesting that around 20-50 myosin proteins are pulling on filaments to move the bead. Yet, when TLP was missing the generated force dropped by 70%. Manipulation of actin filaments can be done in different ways and we used small molecules that either stabilize or disrupt filaments and also generated a parasite lacking one of the few actin filament binding proteins that the parasite genome encodes: coronin. Parasites that were disturbed in this way showed less efficient motility and lower force generation. Also coronin(-) parasites showed a defect in progression along the parasite life cycle as they were less efficiently entering into mosquito salivary glands. Generation of a number of parasite lines with targeted amino acids replacements and experiments using known kinase inhibitors and calcium chelators revealed a signalling cascade from parasite activation to actin filament formation and disassembly, where coronin appears to play a key part in organizing actin filaments.
In our renewed HFSP Young Investigator Grant we will now investigate mutations in actin and myosin to further dissect how forces are generated during malaria parasite motility, keeping in mind that the divergence of the two proteins compared with the myosin and actin in our body might eventually yield a small molecule that slows down the parasites before they enter into our blood.
References
1. Coupling of Retrograde Flow to Force Production During Malaria Parasite Migration. Quadt KA, Streichfuss M, Moreau CA, Spatz JP, Frischknecht F. ACS Nano. 2016; 10(2):2091-2102.
2. The actin filament-binding protein coronin regulates motility in Plasmodium sporozoites. Bane KS, Lepper S, Kehrer J, Sattler JM, Singer M, Reinig M, Klug D, Heiss K, Baum J, Mueller AK, Frischknecht F. PLoS Pathogens. 2016; 12(7): e10057.
Link to PLoS Pathogens article


































