Cell fate assignation through asymmetric cell division contributes to the plethora of cell types of an organism. Differential cell fate assignation after division relies on the biased dispatch of cortical fate determinants among the two daughter cells.
In recent years, novel mechanisms of asymmetric dispatch of cell fate determinants have been found to operate in the cytoplasm, away from the cell cortex. In particular, it has recently been shown that Sara (Smad anchor for receptor activation) signaling endosomes also segregate asymmetrically during the asymmetric division of Drosophila Sensory Organ Precursors. Since Notch and its ligand Delta traffic through these endosomes, this asymmetric endosome motility contributes to asymmetric Notch/Delta signaling and thus to asymmetric cell fate determination. In flies, this has been generalized to stem cells in the gut and in the central nervous system, as well as to neural precursors of the spinal cord in zebrafish. Our work now unravels the molecular and physical mechanism underlying this asymmetric endosome motility event (see figure).
Click on image to enlarge
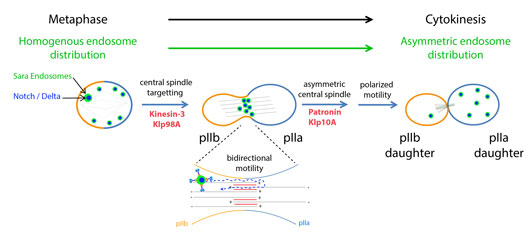
Figure: Proposed mechanism controlling asymmetric dispatch of signaling endosome during the asymmetric division of Drosophila Sensory Organ Precursors.
Through robust quantitative analysis of Sensory Organ Precursor divisions, we found that the asymmetric segregation of Sara endosomes can be explained by the activity of only three proteins. First, the plus-end kinesin motor Klp98A targets Sara endosomes to the central spindle, the microtubule scaffold specific to anaphase. At the central spindle, endosomes move bidirectionally on an antiparallel array of microtubules. Simultaneously, the microtubule depolymerising kinesin Klp10A and its antagonist Patronin generate central spindle asymmetry, in a way that within the antiparallel bundle, more microtubules are pointing towards the pIIa daughter cell compared to the pIIb. This asymmetric spindle, in turn, polarizes endosome motility, ultimately causing asymmetric endosome dispatch to the pIIa daughter cell. To demonstrate this mechanism, we inverted the polarity of the spindle by targeting Patronin asymmetrically to the cortex using nanobodies. This spindle inversion causes endosomes to be delivered to the wrong cell.
To explain quantitatively how spindle asymmetry controls endosomes asymmetry, we developed a theory of the motility of endosomes in an asymmetric microtubule antiparallel overlap. Importantly, this theory defines a very simple equation explaining quantitatively this complex cellular behavior.
Altogether, our results uncover a mechanism by which cytosolic cargoes in general, and signaling endosomes in particular, can be targeted to only one of the daughter cells during asymmetric division. Moreover, our theory can readily be applied beyond asymmetric division to explain polarized traffic in other microtubule antiparallel overlaps, for instance in vertebrate dendrites.
Reference
Polarized endosome dynamics by spindle asymmetry during asymmetric cell division. Derivery E, Seum C, Daeden A, Loubéry S, Holtzer L, Jülicher F, Gonzalez-Gaitan M. Nature. 2015 Dec 10;528(7581):280-5.