Theories usually consider an ensemble of networks of different sizes and study their behaviors as the number of neurons tends to infinity. This technique called “scaling” enables general variables such as the firing rate or the correlation between neurons from network parameters to be derived analytically. Importantly, the strength of synaptic connection J must scale as the inverse square root of the number of connections per neurons K (J=1/√K). Theories also use networks composed of homogenous population of neurons that are stimulated uniformly. Whether these assumptions hold under biological conditions is unclear: validation requires accurate information about the network architecture and the precise control of the input to the network. This is not possible with in vivo preparations, which in addition have too many uncontrolled variables (e.g. state of the animal, anaesthesia).
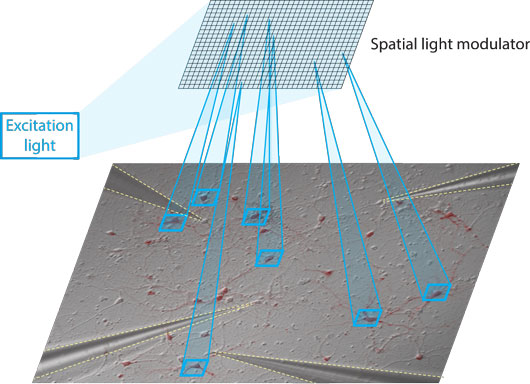
Figure: Optogenetic stimulation of the network. A spatial light modulator was used to generate patterns of light and to stimulate a subpopulation of neurons that express channelrhodopsin (red). Four glass electrodes were used to record membrane potential and spikes in non-stimulated neurons.
HFSP Long-Term Fellow Jérémie Barral found an ingenious way to test the scaling hypothesis, using in vitro cultures of cortical neurons. Because each individual neuronal culture has a different cell density, these cultures provide a means to test the scaling laws assumed by statistical mechanics-based models of cortical circuits. Amazingly, synaptic strengths in the cultured networks do actually obey the inverse square root law. Using patterned optogenetic stimulation, the authors then show that the theoretically predicted dynamics occur under physiological conditions far from the assumed asymptotic limits. Moreover, salient features of stimulus-evoked neural responses in vivo are also present in the cultures.
Confirmation of the scaling rule, which has been under debate, will allow theorists to develop models that are more in line with the biology. That the predicted responses hold under physiological conditions effectively bridges theory with experiments and can potentially provide a foundation for interpreting the countless data that are being collected under the brain initiative and connectomics. Finally, the finding that many of the responses evoked in generic cultures resemble those observed in vivo is surprising because a network of cultured neurons is neither a computer simulation nor an intact brain. This suggests that the synaptic scaling rule and resultant dynamics are emergent properties of networks in general.
Reference
Synaptic scaling rule preserves excitatory–inhibitory balance and salient neuronal network dynamics. Barral J and Reyes AD. Nature Neuroscience. 2016, doi:10.1038/nn.4415.


































