Chloroplasts (plastids) arose from a cyanobacterial endosymbiont and have retained their own genome. Consistent with their bacterial origin, chloroplasts multiply in the host cytosol by binary division of pre-existing chloroplasts, which is mediated by a duplex ring complex called the plastid-dividing (PD) machinery (Figure 1). The PD machinery includes inner ring structures (FtsZ ring) and outer ring structures (PD ring and dynamin ring) that form at the chloroplast division site and dissect the chloroplast into two daughter chloroplasts. Chloroplast FtsZs are tubulin-like GTPases that descended from a precursor in the cyanobacterial ancestor of chloroplast. In bacteria, FtsZ proteins assemble into a single ring beneath the cell membrane and provide the contractile force to drive bacterial cell division. Interestingly, whereas bacteria have one FtsZ gene in their genomes, the chloroplast FtsZ gene was duplicated and these duplicated loci, now present in the nuclear genomes, are widely conserved throughout photosynthetic eukaryotes. However, the mechanisms of FtsZ-ring assembly and constriction in the PD machinery and the functional significance of the duplication and retention of two FtsZs (FtsZ1 and FtsZ2) during chloroplast evolution are not fully understood.
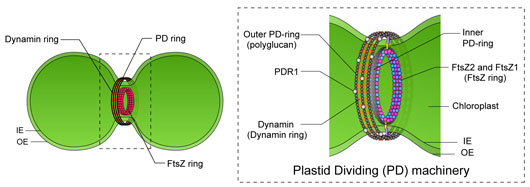
Figure 1: Schematic representations of dividing chloroplast and PD machinery.
In this study, to investigate the molecular mechanisms of the FtsZ ring, we expressed fluorescently tagged Arabidopsis thaliana FtsZ1 (AY113896) and FtsZ2 (AF089738) in the spherical cells of the yeast P. pastoris. As a result, both FtsZ1 and FtsZ2 assembled into a single, closed ring in Pichia cells. Furthermore, we determined that co-expressed FtsZ1 and FtsZ2 could co-assemble into one uniform ring, in which the stoichiometry of FtsZ2 and FtsZ1 was approximetly 1:1 (Figure 2, left). Chimaeric chloroplast FtsZ proteins, Z2-Z1 and Z1-Z2, in which the amino (N)-terminal halves of FtsZ1 and FtsZ2 were swapped to mimic the interfaces of putative heterodimers of FtsZ2 and FtsZ1 revealed the hetero-interaction of both FtsZ2-FtsZ1 and FtsZ1-FtsZ2 by head-to-tail cross association (Figure 2, right). In addition, competitive binding experiments using polymerization-deficient mutants of FtsZ2 and FtsZ1 showed that the chloroplast FtsZ ring predominantly assembles by heteropolymerization.
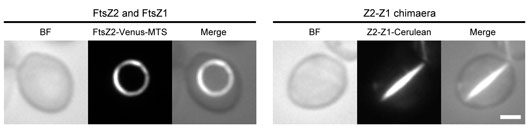
Figure 2: Fluorescence images of a co-assembled FtsZ ring and a filament bundle of Z2-Z1 chimaera proteins in the P. pastoris cells. Scale bar, 1 μm.
Taken together, we proposed the following assembly model of the chloroplast FtsZ ring; first, free monomers of FtsZ2 and FtsZ1 assemble FtsZ2-FtsZ1 and FtsZ1-FtsZ2 heterodimers and heterooligomers. Then, when the concentration of FtsZ2-FtsZ1 and FtsZ1-FtsZ2 dimers and oligomers reaches a critical threshold, they rapidly assemble into bundles of FtsZ protofilaments via tubulin-like heteropolymerization and form the ring. Furthermore, we found that the reconstituted FtsZ rings can constrict in the Pichia cells and the co-assembled ring constricted more rapidly than the single-assembled ring. Although the molecular mechanism remains unclear, we presumed that a decrease in average protofilament length and increasing of protofilament turnover promote contraction of the FtsZ ring.
Thus, the synergistic kinetics of FtsZ1 and FtsZ2 are likely to enable innovation of the functions of chloroplast FtsZs for the chloroplast division mechanism, altering filament mobility, size and flexibility compared with those of bacterial FtsZ. In spite of the low level of sequence similarity between FtsZ and tubulin, it is known that they have significantly similar three-dimensional structures. Therefore, our results also suggest that gene duplication led to convergent evolution in the ability of chloroplast FtsZs and eukaryotic tubulins to form, although assembly mechanisms differ, structurally similar protofilaments involving hetero-interaction by two distinct protein molecules.
Reference
Chloroplast FtsZs assembles into a contractible ring via tubulin-like heteropolymerization. Yoshida Y, Mogi Y, TerBush AD, Osteryoung KW. Nature Plants. 2016 June 20; 2, Article number: 16095 (2016). doi: 10.1038/NPLANTS.2016.95.


































