We first engineered an optimized near-infrared voltage sensor called QuasAr3 which allowed high fidelity recording of action potentials in acute brain slices. Using a Cre-dependent transgenic mouse line expressing both QuasAr3 and the channelrhodopsin CheRiff we performed all-optical classification of neuronal subtypes based on their optically induced firing patterns. We also developed a photoactivated variant (paQuasAr3), which showed brighter fluorescence and improved signal to noise ratio upon activation with blue light. Sparse expression of paQuasAr3 reported spontaneous spiking activity in the hippocampus and olfactory bulb of anesthetized mice and allowed us to record the details of voltage propagation at subcellular resolution, in vivo.
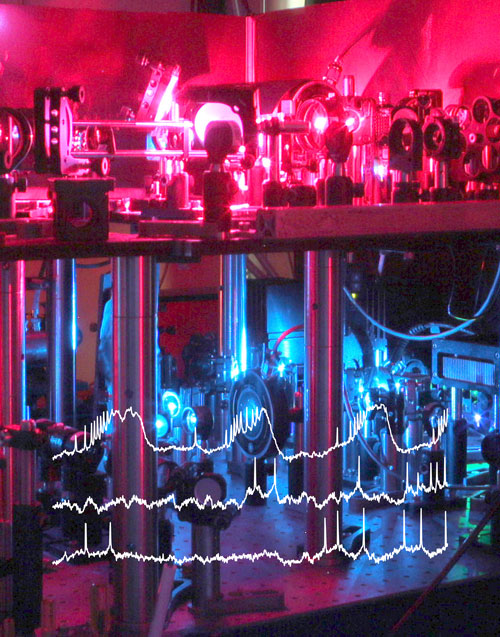
Figure: Optical setup for voltage imaging and optogenetic control. Overlayed traces show examples for optical intracellular recordings from 3 cells in the hippocampus of a behaving mouse. Photo: Dr. He Tian.
To record and manipulate multiple cells simultaneously we next developed soma restricted versions of QuasAr3, paQuasAr3 and CheRiff which enabled dense expression by significantly reducing the neuropil background. To further improve the imaging contrast, reduce the overall laser power and allow precise spatial control, we designed a new microscope which enabled patterning of the stimulation and imaging lasers with a digital micromirror device. Using the combination of dense expression and patterned illumination we recorded spikes and subthreshold dynamics from multiple cells, simultaneously in the hippocampus of awake, behaving mice.
Using this technology, we imaged inhibitory and excitatory cells and compared their activity during resting and walking. We revealed behavior and cell-type dependent changes in the firing rates, intracellular oscillations and subthreshold correlations between multiple cells. In combination with stimulation using optogenetics, we revealed changes in neuronal excitability that were dependent on the behavioral state, reflecting the interplay of excitatory and inhibitory synaptic inputs. This set of tools opens the possibility to decipher neuronal circuits at high spatiotemporal resolution and unprecedented throughput.


































