Bioelectronic devices allow us to record and stimulate different phenomena in our bodies down to the single cellular level. However, as with any implantable structure, the placement of these devices typically initiates a scarring and encapsulation response, which limits the capabilities of the implant long-term. In this study, the research developed by Alexander J. Boys, HFSP Fellowship Awardee 2020, developed an implant design to mitigate and reduce the degree of this response, showing, in some cases, that it can eliminate this response altogether.
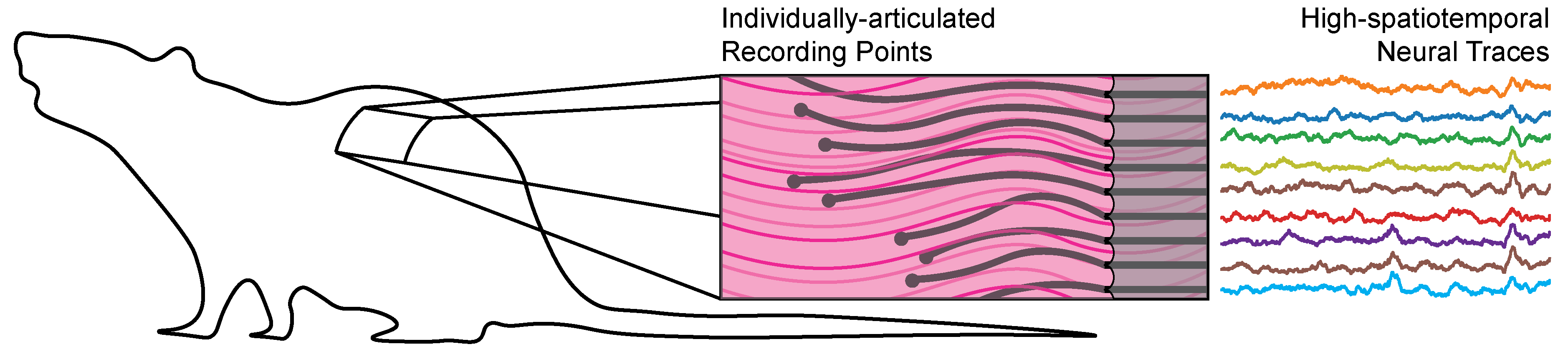
The design strategy used by the researchers involved a hybrid approach combining aspects from microelectronic fabrication with principles from tissue engineering and regenerative medicine to produce a structure that promotes tissue ingrowth around the implant during the repair process. They created a neural recording device consistent with individually-articulated, subcellular-sized wires and then embedded these wires inside of a resorbable hydrogel, designed to mimic tissue micro-architecture. The researchers from the Department of Chemical Engineering and Biotechnology University of Cambridge, UK, placed these devices into the musculature of rats to understand and map out the healing response of tissue to these implants. Over the course of 2 months, the resorption of the gel was observed, resulting in a close interface between the bioelectronic devices and the surrounding tissue. Then, the team used this resultant placement to record electromyographic signals, finding evidence for the recording of individual motor units in a long-term implant setting. The results also show that these implants can be used in other contexts, through the addition of live cells into the implant structure, as well as the placement of the original implant concept into the brain.
These devices provide cellular-level recordings at a high spatiotemporal resolution in free-moving animals with little to no scarring of encapsulation of the implant. These types of devices are useful for the production of neuro-prostheses in amputees, where a prosthetic limb or similar is driven by local neural impulses that would typically innervate the lost-limb. The reduction in the tissue response to the placement of our devices offers access to high-resolution data, on the scale of individual motor units within a singular muscle group. These types of implants can help drive a new paradigm in the production and treatment of severe and traumatic injuries through the utilization and discovery of neural information at the cellular-level.


































