Under the cell membrane, a thin meshwork of actin filaments and myosin molecular motors, the cell cortex, acts as a scaffold and determines the cell shape. This material is dynamically organized and responds to several biochemical and mechanical signals in order to modify the cell architecture. During cytokinetic ring assembly, we explored the role of myosin-induced flows of cortical actin filaments observed from the pole towards the cell equator. By the use of active nematic gel theory, a theory coming from liquid crystals and describing the orientation of rod like objects, we identified the physical mechanism coupling these compressive flows and actin filaments alignment and determined the emergent cortical material parameters.
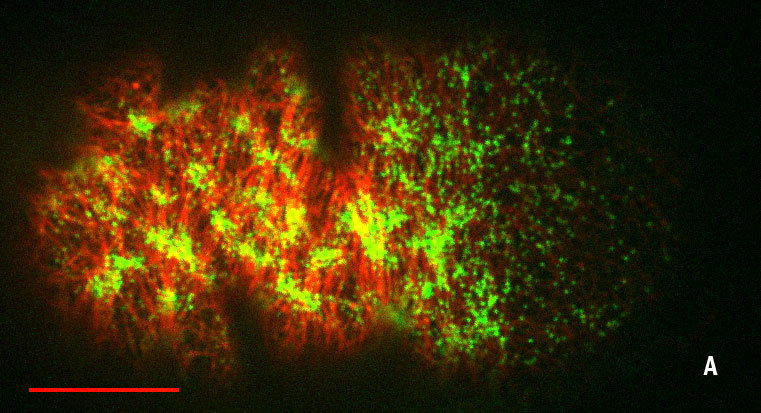
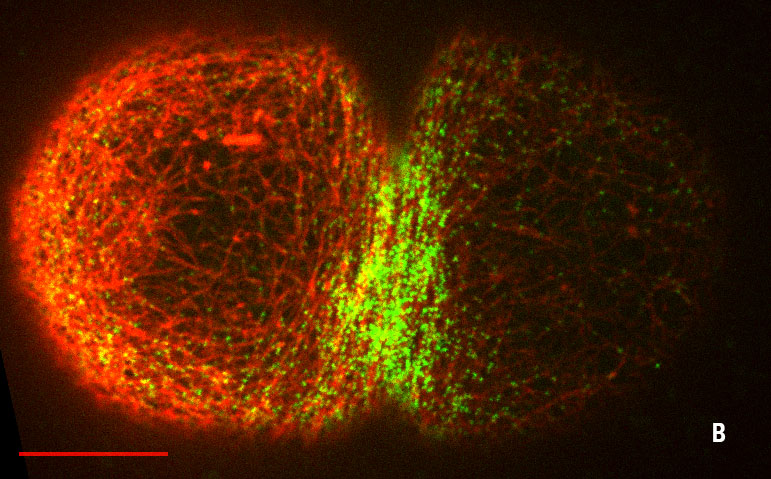
Figures: Cortical organization during the two independent equatorial ring constrictions of the C. elegans zygote, pseudocleavage (fig. A) and cytokinesis (fig. B) (Lifeact in red, myosin in green, scale bars 10 μm).
We characterized flow-alignment coupling, and verified at a quantitative level that compression by flow drives ring formation with or without local active alignment by molecular motors. We discovered that cortical flows trigger the assembly of a transient contractile ring in the C. elegans one-cell embryo, the pseudocleavage furrow, occuring prior to cytokinesis and in the absence of local molecular motor recruitment. This study highlights, once more, the important role of mechanical forces in the dynamical complex organization of cytoskeletal architectures.
Reference
Cortical flow aligns actin filaments to form a furrow. Anne-Cecile Reymann, Fabio Staniscia, Anna Erzberger, Guillaume Salbreux, Stephan W Grill, eLife, October 10, 2016. DOI: http://dx.doi.org/10.7554/eLife.17807


































