Replication of biological molecules is the foundation of life, and it usually requires complex cellular machinery. However, a number of disease-related processes involve self-replication of protein assemblies without any additional assistance. A striking example is the self-replication of amyloid fibrils that are implicated in a number of neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases. These fibrils, when intertwined and entangled with each other, form the plaques that are found in the brains of Alzheimer’s patients.
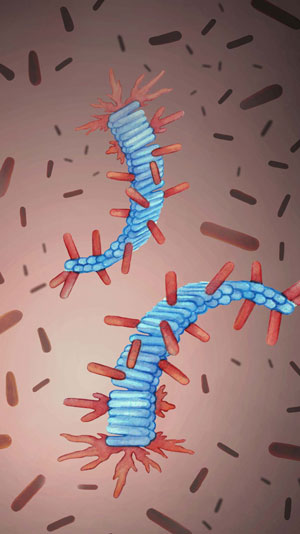
Figure: Artist’s rendering of protein fibrils from computer simulations (in blue) and healthy proteins deposited on them (in red).
Spontaneous formation of the first amyloid fibrils is very slow, and it typically takes decades. However, once the first fibrils are formed, they begin to replicate and spread much more rapidly by themselves. This positive feedback makes the related diseases extremely challenging to control.
Using a combination of computer simulations and biophysical experiments on Alzheimer’s Aβ peptide, we found that the process of the fibril self-replication is governed by build-up of healthy proteins on the surface of existing fibrils. When this build-up reaches a certain amount it triggers fibril self-replication, at which point the rate of production of new fibrils and small protein aggregates explodes. This process is extremely sensitive to how the healthy protein interacts with its fibrils, which results in its high specificity and sensitivity to environmental conditions. As a proof of principle, we further showed that by changing how the healthy proteins interact with the surface of fibrils, it is possible to control the fibril self-replication.
Our study suggests that by controlling the build-up of healthy proteins onto the surface of fibrils, the spread of the pathological protein aggregates could be slowed down. The results of this work could also be of great interest for bio-nanotechnology, where one of the unfulfilled goals is achieving self-replication in manufacturing of bio-nanomaterials. Learning the design rules from the fibrils that self-replicate naturally may help achieve this goal.
Reference
Physical determinants of the self-replication of protein fibrils. A. Saric, A. K. Buell, G. Meisl, T. C. T. Michaels, C. M. Dobson, S. Linse, T. P. J. Knowles, D. Frenkel. Nature Physics; 18 July 2016; DOI: 10.1038/NPHYS3828.


































