The amazing variety of shapes that different types of cells acquire reflects the complex interplay between biochemical regulation - by genes and proteins - and mechanical constraints – provided by the biophysical properties of cells and their environment. In this paper, we have imaged the dynamic behaviour of growth regulatory proteins in fission yeast, a cylindrically-shaped unicellular eukaryote, and incorporate these molecular data within a mechanical model of cell wall growth, in order to investigate how the specific geometry of cell growth domains emerges. We find that the pattern of exocytic vesicle delivery plays a dominant role in shaping cell growth domains and that cell wall mechanics and exocytic pattern suffice to predict growth domain morphogenesis throughout the entire cell cycle in this species.
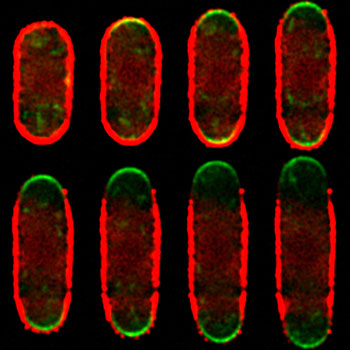
Figure: Time-lapse image sequence of a growing fission yeast cell expressing the exocytically-delivered reporter of glucan synthesis Bgs4-GFP (green) and whose wall was decorated with fluorescent quantum dots (red). Quantum dots can be seen splaying out as the cell wall expands at the polar areas of active cell growth.
Why this is surprising is because, although exocytosis is known to play a crucial role in cellular growth, it had not been involved in cell shape definition, which is typically thought to rely on control by other machineries like the cytoskeleton and Rho-like GTPases such as Cdc42. Instead, we demonstrate in fact that forceful re-directioning of exocytic vesicle fusion to atypical areas of the cell cortex induces precisely proportional shape changes to growth domains, demonstrating that both features are causally linked in the case of this species. We believe these findings will help shift the focus of future morphogenesis studies from the role of the cytoskeleton and Rho GTPases and the processes they control, to the role of the specific processes controlling tethering and fusion of vesicles with the plasma membrane, opening exciting interdisciplinary biophysical and cell biological avenues of future research.
Reference
Wall mechanics and exocytosis define the shape of growth domains in fission yeast. Abenza JF, Couturier E, Dodgson J, Dickmann J, Chessel A, Dumais J, Salas RE. Nat Commun. 2015 Oct 12;6:8400. doi: 10.1038/ncomms9400. PMID: 26455310. PMCID: PMC4618311.


































