The pore-forming α-subunit of sodium channels can associate with multiple protein building blocks, one of which is called the β2 subunit. Until now, it was not known exactly how these two partners come together to form a channel complex. However, previous studies did suggest which regions of the two building blocks make contact with each other.
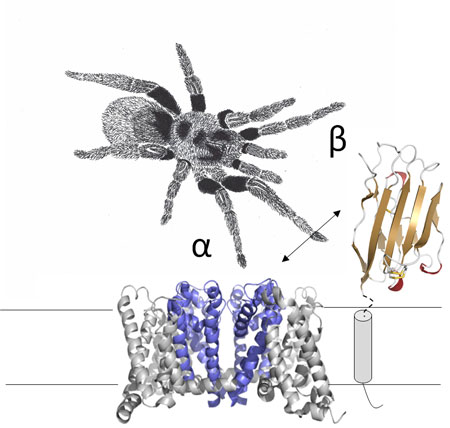
Figure: Shown is an illustration depicting a sodium channel α-subunit model next to the β2-subunit extracellular structure attached to a symbolized version of the transmembrane segment. The tarantula sketch signifies the ability of spider toxins to sense the interaction between α- and β-subunit.
Das, Gilchrist et al. have now visualized the 3D structure of the β2 subunit in very high detail by using X-ray crystallography. The level of detail in the structure allowed the identification of amino acids that make up the region that anchors to the sodium channel. Next, the team mutated three amino acids in the sodium channel itself, and treated frog eggs containing the mutant channel with a tarantula toxin that binds between the α- and β-subunit. By doing so, they revealed the location and identity of the exact anchoring point between both proteins. In particular, they found that Cys at position 55 in the β2 subunit forms a disulfide bond with Cys at position 910 in the sodium channel domain II pore loop, thereby suggesting a 1:1 stoichiometry. Finally, their results also reveal which disulfide bonds are formed between adjacent 912/918Cys residues. The concepts emerging from their work yield precious information on how both partners cooperate to form the sodium channel complex and may be used to provide mechanistic insights into mutations that cause cardiac disorders and epilepsy syndromes.
Reference
Binary architecture of the Nav1.2-b2 signaling complex. Samir Das, John Gilchrist, Frank Bosmans, Filip Van Petegem. eLife 2016;5:e10960. DOI: 10.7554/eLife.10960.


































