Precisely knowing the stoichiometry of their components is critical for investigating structure, assembly, and function of macromolecular machines. This has remained a technical challenge in particular for large, hydrophobic membrane-spanning protein complexes. Bacterial type III secretion systems are one kind of cell envelope-spanning effector protein-delivery machine essential for colonization and survival of many Gram-negative pathogens and symbionts. The core unit of these secretion systems, termed needle complex, is composed of a base that anchors the machinery to the inner and outer bacterial membranes, a hollow needle that serves as conduit for substrate proteins, and a membrane-embedded export apparatus facilitating substrate translocation. In total, these large systems are composed of up to 20 different proteins with one to several hundred copies each. Structural analyses have revealed the buildup of the components of the base, but the stoichiometry of the essential hydrophobic export apparatus components remained unknown.
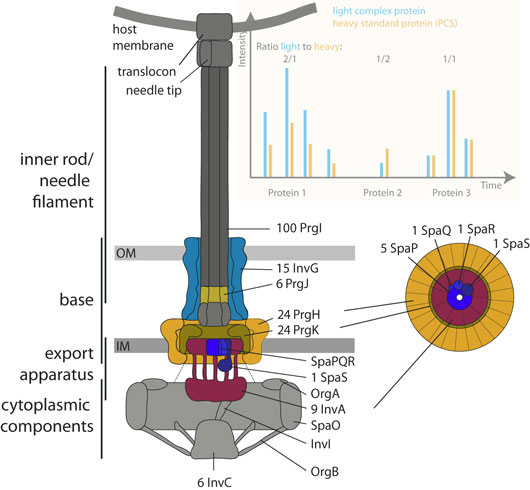
Figure: Mass spectrometry-based stoichiometry determination of the type III secretion system's needle complex.
We have developed two complementary protocols for gentle purification of complete needle complexes and adapted peptide concatenated standard and synthetic stable isotope-labeled peptide-based mass spectrometry to determine the stoichiometry of a type III secretion system of the food-borne enteropathogen Salmonella enterica serovar Typhimurium. In this way, we were able to show that the export apparatus of type III secretion systems contains five copies of the protein SpaP, one SpaQ, one SpaR, one SpaS, and nine InvA, and that the inner rod of these systems is merely a rod and is just composed of six subunits of the protein PrgJ.
Providing this structural information will facilitate efforts to obtain an atomic view of these and of the related flagellar systems and as such help to develop anti-infective strategies targeting these machines central to the virulence of many pathogens. In addition, the newly developed extension of mass spectrometry-based stoichiometry determination to investigate highly hydrophobic complexes of very heterogeneous composition and wide stoichiometric range may foster the analysis of other membrane-spanning protein complexes, in particular of different virulence-associated bacterial secretion systems.
Reference
Determination of the Stoichiometry of the Complete Bacterial Type III Secretion Needle Complex Using a Combined Quantitative Proteomic Approach. Susann Zilkenat, Mirita Franz-Wachtel, York-Dieter Stierhof, Jorge E. Galán, Boris Macek, Samuel Wagner. Molecular & Cellular Proteomics 15: 1598–1609, 2016.


































