When we move, eat or fall in love, internal movements of our muscles, gut and cardiovascular system generate mechanical forces that are sensed by specialized receptor cells that communicate these shape changes to the brain. With the discovery of the PIEZO ion channels, these mechanosensitive body-brain axes recently received considerable momentum, but the endogenous force transmission pathway leading to mechanoreceptor activation is largely unknown, limiting the conceptual advance of proprioception and visceral mechanosensation. This is due in part to the difficulty to record from visceral mechanoreceptors embedded deep within an animal’s body, especially in moving animals, and much of our knowledge comes from restrained and paralyzed animals, or tissue explants.
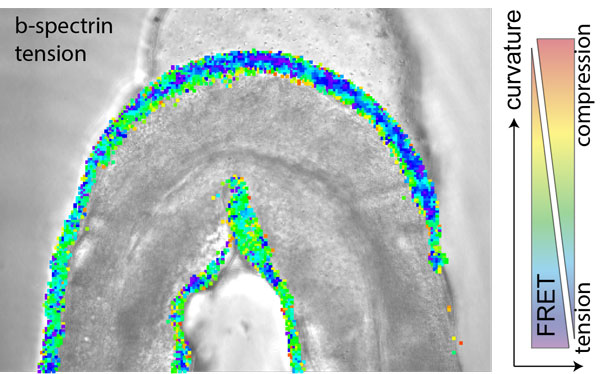
Figure: A single animal expressing a genetically encoded tension sensor is confined in a microfluidic channel with varying curvature and shows varying mechanical stress along the concave and convex body bends. Colour scale represents FRET values derived after image processing and is over layed with the brightfield image of the trapped animal.
Traditionally, the neurons and receptor cells involved in initiating proprioception and related sensory modalities are commonly referred to as `stretch’ receptors. Many of these sensory cells, however, have long processes and axons that are subjected to stretch and compression at the same time at different locations. This is often observed in the small round worm Caenorhabditis elegans, in which a single axon can span the entire length of the animal. Consequently, and due to the rhythmic undulations inherent with forward crawling in this worm, such axons experience opposite mechanical stresses at any given moment in time such that they locally elongate and shorten at the points of highest curvatures.
How is this complex mechanical information decoded such that it can be used to coordinate something as harmonic as C. Elegans’ crawling? Research in the Krieg lab, funded by an HFSP Career Development Award, has now shown that the proprioceptor activity of a single neuron, called DVA, peaks under neuronal compression in vivo. This is in contrast to the well accepted stretch receptor paradigm, in which neurons preferentially activate under mechanical extension. Rather, by stretching single neurons on an elastic substrate or their membrane directly with an optical tweezer apparatus, the researchers found that the neuron DVA seemingly deactivated under mechanical extension, which was visible by a reduced calcium signal in the stimulated axons. The same signatures were also visible in freely moving animals, in which the proprioceptor DVA activated specifically during ventral bends, when the sensory axon is under mechanical compression. However, when the axon cannot sustain mechanical compression, e.g., in mutations of the neuronal b-spectrin cytoskeleton, in which the axons buckle, the DVA activity is strongly reduced, concomitant with a failure to accurately delimit their body posture. With the help of genetically encoded sensors for mechanical tension, the researchers could also show that the spectrin cytoskeleton can sustain and transmit mechanical compression, in addition to its previously known role in sustaining mechanical prestress. At the end, the researchers also found molecular representatives for the mechanical electrical transduction and could show that the activity increases under compression, partially dependent on a mechanosensitive ion channel TRP-4 NOMPC, while the decrease in neuronal activity under tension was abolished in mutants for the two-pore potassium channel TWK-16/ TREK2.
The implications go beyond mechanosignaling in the worm, pointing towards a more general principle for how mechanical stresses compartmentalize neuronal activity in long axons (through mechanosensitive ion channels) that are subjected to varying curvatures at a given time, e.g., during body movement or organ distension. Similar mechanisms could be at play in the mechanosensitive processes of the vagus nerve.
The support of the HFSP Career Development Award enabled a particular aspect in this research that helped the scientists to establish high-resolution force measurement assays based on optical tweezer technology and microfluidics.
|
HFSP award information Career Development Award (CDA00023/2018-C) - Project: A prosthetic photon-based neurotransmitter system to overcome synaptic transmission barriers |


































