When Mike Barry, a former colleague at MIT, handed me the Heterosigma akashiwo cell culture, it came with his personal ‘good luck’ note. Before long I realized why luck would come handy in tracking the migratory behaviour of H. akashiwo, which for years has served as a model species to study gravitaxis – the tendency of cells to swim along or against the gravity force. As I was getting started with the H. akashiwo, one of the numerous phytoplankton species which abound our oceans, the unexpected surfaced rather soon – a fraction of swimming population rapidly switched its direction when briefly exposed to turbulent cues. The emergence of a behaviourally distinct subpopulation was an ample hint that there could be more to these microorganisms than what had been reported until then. Thus began the exhilarating journey to decode how phytoplankton species like H. akashiwo navigate through dynamic aquatic environments. The search for the answer has, to date, taken us to the seas and to the skies (a sequel to this story was shared recently), and only now, we are beginning to connect the first dots of the grand puzzle that some of Earth's most important organisms have presented us.
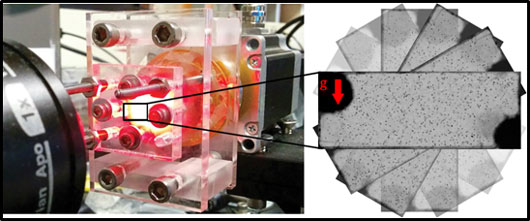
Figure 1. Ocean-in-a-lab. The automated millifluidic chamber used to expose phytoplankton to turbulence-like reorientations. g indicates the direction of the gravity force.
Phytoplankton are microscopic photosynthetic organisms which form the base of most aquatic food webs, regulate the global biogeochemical cycles, and by transferring CO2 from the atmosphere to the ocean, produce half of the world’s oxygen. Many species of phytoplankton are motile and often migrate in response to gravity: upwards toward light during the day, and downwards at night, toward higher inorganic nutrient concentrations. Disruption of this diurnal migratory strategy by turbulence is considered to be an important cause of the succession between motile and non-motile species when conditions turn turbulent. Ramon Margalef, one of the founding fathers of modern marine biology, was among the first to realize the fundamental role of turbulence in phytoplankton ecology, which together with nutrients and light, determines the ecological niches in the marine environment. This classical view however did not account for the possibility that motile species may actively respond to turbulent cues, and rapidly adapt to a turbulent landscape.
As phytoplankton shuttle between the sunlit surface waters and the nutrient-rich deeper waters, they are frequently exposed to the turbulent layers, which can potentially disrupt this indispensable migratory behaviour. It is still a mystery how these minute organisms, about a tenth the size of human hair, can navigate through these, often detrimental, turbulent environments. Cells are whirled around by eddies – particularly by the smallest, millimeter-sized flow vortices – in which they get trapped. As an unavoidable fall out, a cell undergoes changes in the direction of gravity relative to its swimming direction, which we captured in our ocean-in-a-lab – a millifluidic chamber programmed to mimic the timescales and statistics representative of ocean turbulence (Figure 1). The chamber could be continuously rotated around a horizontal axis by a computer-controlled motor, mimicking the overturning of cells in the ocean by the smallest eddies. While the hydrodynamic environment in the flip chamber is not equivalent to turbulence, it captured the overturning of cells by small-scale turbulent eddies. Without rotation, cells swam strongly upwards (negative gravitaxis), accumulating at the top of the chamber. However, repeated overturning caused a striking departure from negative gravitaxis. Within minutes, an upward-swimming population split into two subpopulations, one swimming upwards and one swimming downwards (Figure 2).

Figure 2. Tackling the turbulent cues. Equilibrium distribution of the phytoplankton H. akashiwo before (left) and after (right) exposure to the turbulent cues. An upward swimming population (cell accumulation at the top of the chamber) splits into two subpopulations (cell accumulation at the top and the bottom of the chamber). The insets show false-colour epifluorescence micrographs of the cells and capture the shape-asymmetry between the top and the bottom populations. Click here for videos of the swimmers in action.
Quantitative analysis of the cell phenotypes, together with a model of cell mechanics, revealed that this emergent behaviour was accompanied by a modulation of the cells’ fore–aft asymmetry (insets in Figure 2). The minute magnitude of this modulation – typically less than a micrometer – was sufficient to invert the preferential swimming direction of the cells. Since cells that experienced turbulent cues exhibited higher levels of stress than those in a stationary chamber, we speculate that the population split could offer advantage for the species in a manner suggestive of evolutionary bet-hedging. By avoiding turbulence, the downward swimming cells pay a momentary price of receiving too little light to carry out photosynthesis, and hence reduced growth. However, crucially, in face of turbulence, the entire population is not lost, rather only half in the extreme case.
The ability of microorganisms to adapt to environmental cues, spanning vastly different time and length scales is a conundrum that has intrigued biologists and physicists alike. Given that 50 % of the species tested were capable of such an active response, the findings could prove pivotal in untangling one of the longstanding challenges in oceanography – the distribution of phytoplankton in the ocean. This study captures one of the most exquisite adaptive strategies in the planktonic world, and highlights that rapid adaptive responses can be important survival strategies for phytoplankton, more so in today’s rapidly shifting environmental and climatic trends.
|
Short biography Anupam Sengupta is an HFSP Cross-Disciplinary Fellow, researching at the broad interface of marine biology, material sciences, fluid dynamics and theoretical ecology. He holds a dual Degree in Mechanical Engineering (integrated Bachelor and Master Degrees) from the Indian Institute of Technology Bombay, India. After a brief stint in industry, Dr. Sengupta moved to the Max Planck Institute for Dynamics and Self Organization, Göttingen, Germany to work on his doctoral research with Christian Bahr and Stephan Herminghaus. Here he received a Ph.D. in physics for his work on Liquid Crystal Microfluidics with highest distinction, summa cum laude. In 2014, Dr. Sengupta switched fields and moved to MIT in Cambridge, USA with the HFSP Cross-Disciplinary Fellowship to work on biophysical processes in marine environments with Roman Stocker. Since October 2015, he is based in Zurich, Switzerland, and continues his research at the Institute of Environmental Engineering, ETH Zurich. |
Reference
Phytoplankton can actively diversify their migration strategy in response to turbulent cues. Anupam Sengupta, Francesco Carrara & Roman Stocker. Nature 543,555–558 (23 March 2017) doi:10.1038/nature21415.


































