Brain metastasis is a common malignancy, predominantly originating from the dissemination of lung, melanoma, and breast cancers. Researchers have previously shown that the tumor microenvironment, including diverse immune cells, is critical for regulating cancer progression in primary and metastatic brain tumors.
The blood-brain barrier is another key tumor microenvironment component formed by endothelial cells, mural cells, astrocytic end-feet, and microglia. Metastasizing cancer cells use different strategies to traverse the blood-brain barrier and subsequently exploit the vasculature to create the blood-tumor barrier. However, the heterogeneity of the major brain metastasis vascular components, specifically endothelial and mural cells, remains poorly understood and was the focus of the study led by Leire Bejarano, HFSP Fellowship Awardee at the Department of Oncology University of Lausanne.
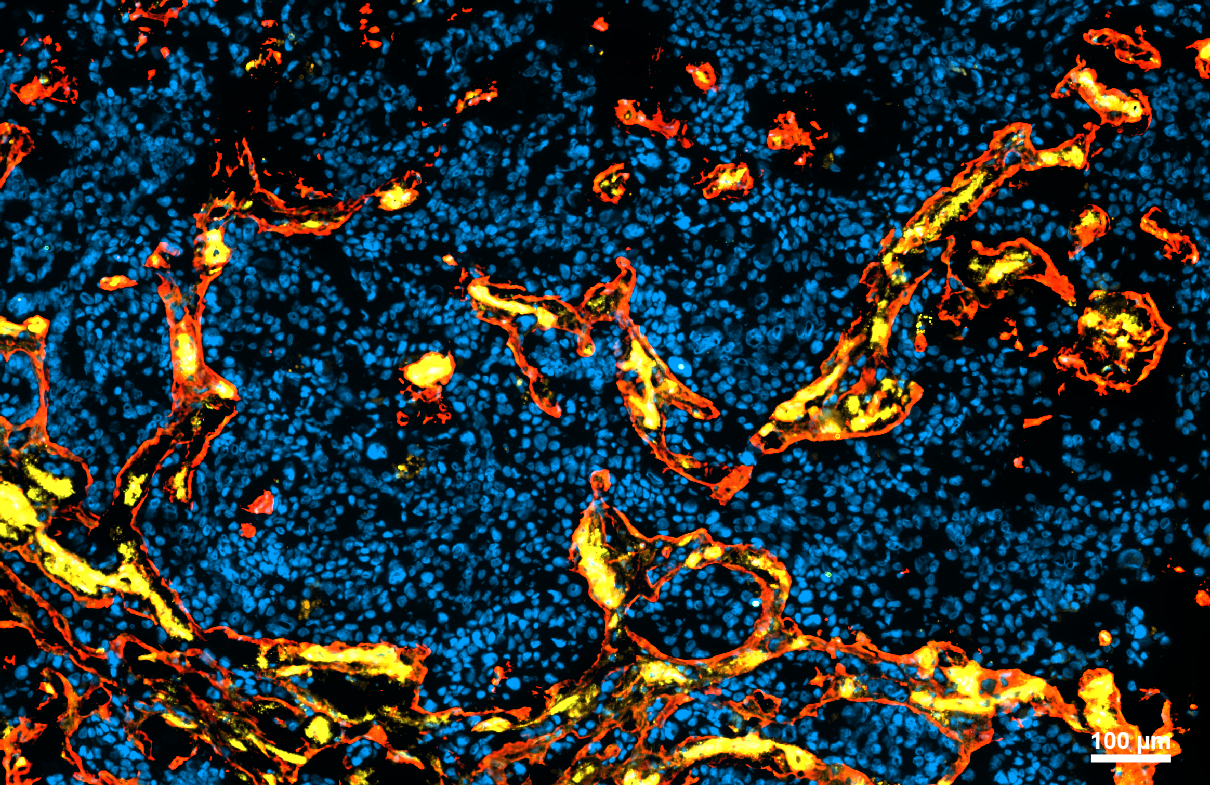
By employing single-cell and bulk RNA-sequencing of the sorted vascular cell types, the scientists’ team identified multiple brain metastasis-enriched vascular subtypes compared to non-tumor brain, including previously unrecognized immune regulatory subtypes. Bejarano and research colleagues integrated the human data with datasets from multiple brain metastasis mouse models, thereby creating a platform to identify vascular targets for the treatment of brain metastasis. The research team found that the CD276 immune checkpoint molecule was significantly upregulated in the brain metastasis vasculature, with anti-CD276 blocking antibodies demonstrating survival benefits in preclinical trials. This study provides important new insights into the complex interactions between the vasculature, immune cells, and cancer cells, with translational relevance for designing innovative therapeutic interventions.


































